What You Should Know:
– 23andMe Holding Co. (Nasdaq: ME), a genetics and biopharmaceutical company, announced The U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for 23ME-01473 (referred to as ‘1473), paving the way for the first human clinical trial of this promising new therapy.
– With FDA clearance secured, 23andMe plans to initiate a Phase 1 clinical trial in the first half of 2024. This initial study will evaluate the safety and efficacy of ‘1473 in patients with advanced solid tumors. This marks a significant step forward in advancing this promising therapy towards potentially helping cancer patients in the future.
Activating Natural Killers: A Novel Approach to Fighting Cancer
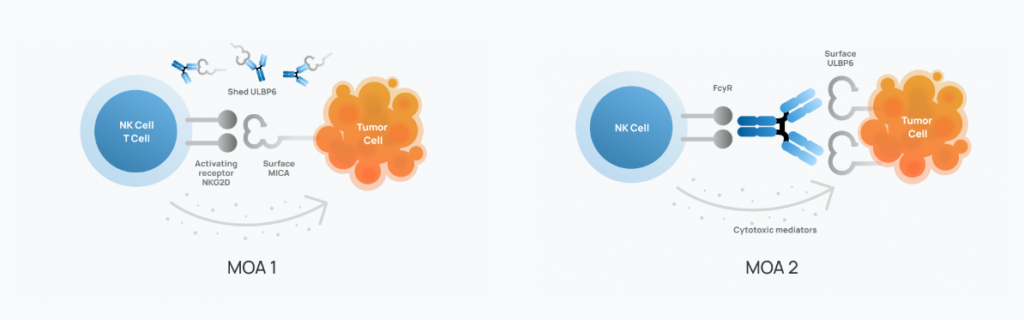
‘1473 represents a unique approach to combating cancer by harnessing the power of natural killer (NK) cells, a key component of the immune system. Unlike traditional immunotherapies that focus on T cells, ‘1473 specifically targets NK cells, offering the potential for broader and more powerful anti-tumor activity.
Targeting a Critical Vulnerability in Cancer Cells
Cancer cells often develop mechanisms to evade immune detection and destruction. ‘1473 works by targeting a specific molecule called ULBP6, which plays a crucial role in this immune evasion strategy. By blocking the interaction between ULBP6 and its receptor on NK cells, ‘1473 aims to restore the immune system’s ability to recognize and eliminate cancer cells.
Dual Mechanism of Action for Enhanced Efficacy
The innovative design of ‘1473 incorporates two distinct mechanisms of action:
- Direct NK cell activation: ‘1473 binds directly to ULBP6, triggering NK cells to attack and kill cancer cells.
- Fc-effector enhancement: This added feature recruits immune system components to further amplify the anti-tumor response.
Addressing the Challenge of Tumor Resistance
One of the major challenges in cancer treatment is the development of resistance to therapies like checkpoint inhibitors. ‘1473’s unique approach, activating both NK and T cells, has the potential to overcome this resistance, offering hope for patients who may not respond to traditional treatments. The upcoming clinical trial will provide crucial data on the safety and efficacy of ‘1473, paving the way for its potential future use in clinical practice.