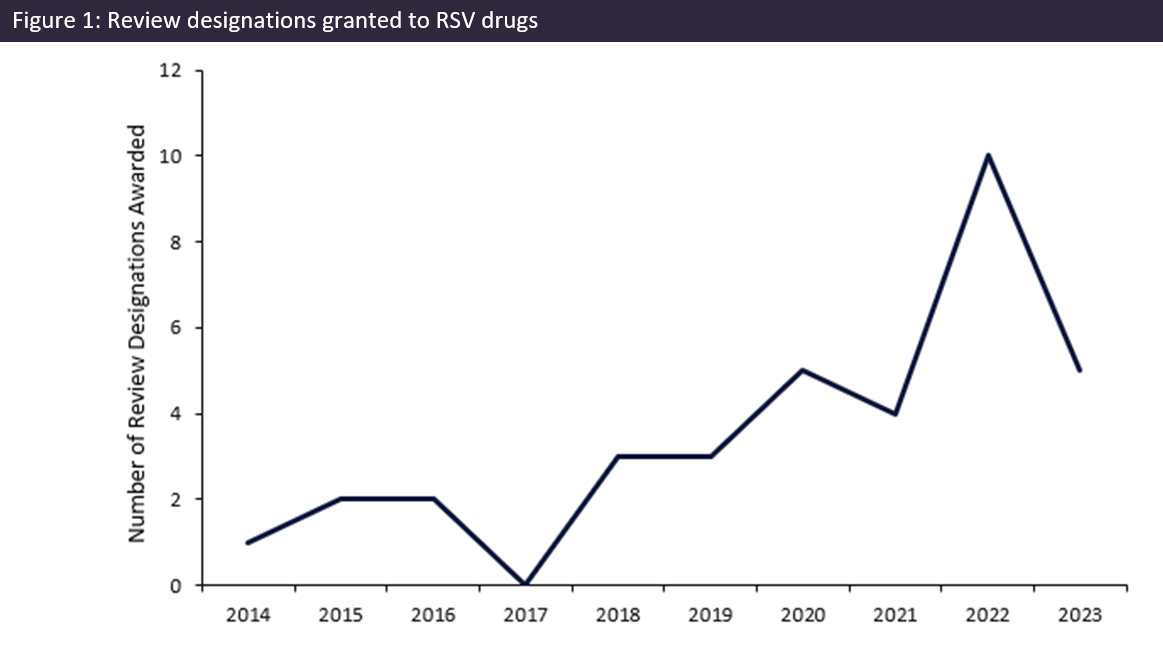
In 2022, global regulatory authorities awarded a record ten review designations for respiratory syncytial virus (RSV)-indicated drugs. This surge acted as a precursor for the RSV drug landscape in 2023, which has seen several novel drug launches, particularly in the field of preventative treatments.
RSV, a common infectious disease of the lungs and respiratory tract, can cause further health problems such as bronchiolitis and pneumonia. Although infections in healthy children and adults are less severe, certain patient groups, such as children with lung disease, the elderly, or the immunocompromised, may experience life-threatening symptoms, with RSV causing 14,000 deaths in the US per year. Until recently, there were no approved prophylactic treatments available.
In 2013, the National Institutes of Health gained the ability to freeze the RSV F protein, which catalysed progress in the development of preventative RSV treatments over the last decade. Regulatory bodies embraced review designations, such as breakthrough therapy, fast track designation, and accelerated approval to promote such treatments and expedite the approval processes.
From 2014 to 2021, the number of RSV drug designations increased steadily, culminating in a significant surge in 2022 (Figure 1). In 2022, 10 designations were granted, representing a 150% increase on the year before. Seven of these designations were awarded by the FDA.
A total of 90% of RSV-related designations in 2022 were granted to prophylactic drugs. Pfizer’s Abrysvo (RSV vaccine), GSK’s Arexvy (RSV vaccine, adjuvanted) and AstraZeneca’s Beyfortus (nirsevimab-alip) accounted for over half of these. These drugs were in later stages of development (Phase II/ III), and were strong candidates for expedited approval.
In 2022, the FDA granted Priority Review to Abrysvo and Arexvy, as well as Breakthrough Therapy to Arexvy. Priority Review directs overall attention and resources to drugs that could significantly improve the prevention of serious conditions when compared to standard applications. Alternatively, Breakthrough Therapy expedites the drug development and review processes for drugs indicated in serious conditions. Both designations enhance the drug approval timelines, expediting vital drugs to the market. By 2023, both drugs received FDA approval, becoming the first two globally approved RSV vaccines.
The recombinant monoclonal antibody, Beyfortus, was granted Accelerated Approval by the EMA. This designation enables the earlier approval of drugs that fill an unmet medical need while treating serious conditions. This preceded its EU and US approvals in late 2022 and mid-2023, respectively, where Beyfortus became the first treatment indicated for disease prevention in infants during their first RSV season.
The upward trend in the number of review designations being granted in recent years, culminating in the record number in 2022, showcases the emphasis by regulatory authorities upon preventative RSV measures. These designations have enabled the earlier approval of life-saving treatments, and GlobalData forecasts the expedited approval timelines of such prophylactic treatments will assist in curtailing RSV hospitalizations and associated fatalities.
