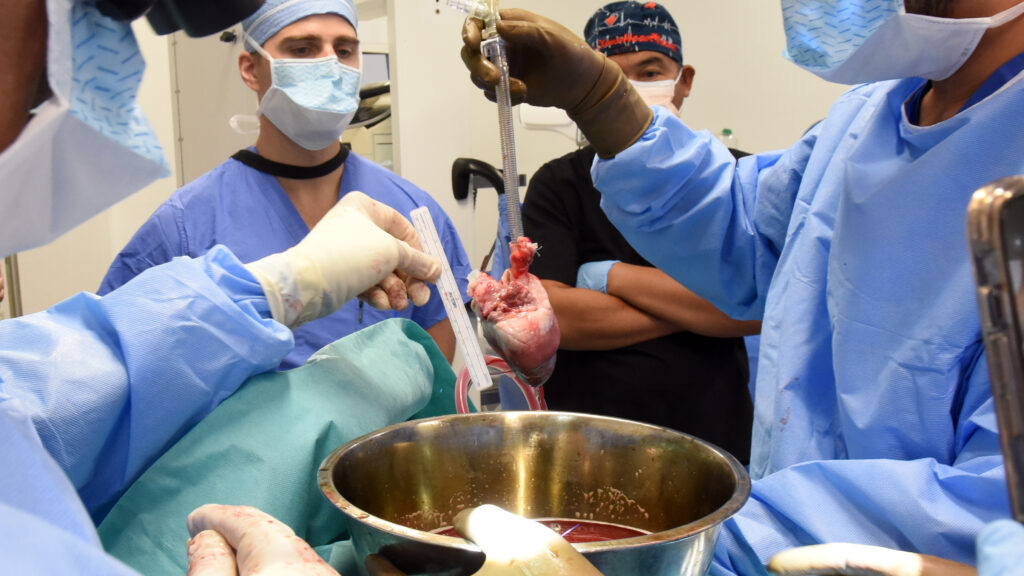
In a new test of xenotransplantation, a medical team at the University of Maryland Medical Center announced Friday that, for only the second time in history, it had transplanted a heart from a genetically engineered pig into a human.
The surgeons performed the eight-hour procedure Wednesday without complications. As of Friday afternoon, the organ recipient, a 58-year-old Navy veteran and former vaccine researcher at the National Institutes of Health named Lawrence Faucette, is awake, able to sit in a chair and breathe on his own, and his new heart is pumping without help from supportive devices, hospital officials said in a statement.
advertisement
Faucette, who lives in Frederick, Md., was admitted to UMMC on Sept. 14, after experiencing heart failure. He was deemed ineligible for a traditional heart transplant because of his preexisting peripheral vascular disease and complications with internal bleeding.
Having survived the first 48 hours with no signs of hyper-acute immune rejection, his doctors are now monitoring him closely for hints of abnormal heart activity, evidence of infection, or signs that Faucette’s body is no longer tolerating the transplanted organ.
“Every day we take it as a victory,” said Muhammad Mohiuddin, who directs the cardiac xenotransplantation program at University of Maryland School of Medicine.
advertisement
But the next few weeks will be critical. Last year, the same medical team performed the first such procedure on a 57-year-old patient named David Bennett. For the first 40 days, Bennett — who had terminal heart failure and was too sick to qualify for a human heart transplant or mechanical assist device — seemed to be recovering. But then he took a turn for the worse and died not long after.
The hearts that both Bennett and now Faucette received came from pigs that have been genetically engineered with 10 changes to their DNA to make their organs better suited to residing within a human body, which include inactivating a growth gene — so the porcine heart won’t continue to expand after transplantation — and other modifications to remove molecules most likely to provoke an immune attack.
The pigs were created by Revivicor, a biotechnology company spun off in 2003 from PPL Therapeutics, the U.K. firm that produced Dolly the sheep, the first mammal cloned from a cell from another animal. In 2011, Revivicor was acquired by United Therapeutics, a pharma company founded and helmed by xenotransplantation enthusiast Martine Rothblatt.
Because the pigs are being raised in a special pathogen-free facility, they are supposed to be virus-free. But University of Maryland researchers revealed last year that traces of a porcine cytomegalovirus had shown up in blood draws taken from Bennett 20 days after his surgery. In an extensive case report published this summer, the Maryland team found widespread damage to Bennett’s blood vessels, likely caused by a destructive inflammatory response to the donor organ. They noted that the porcine virus — which had been lying dormant in the animal and thus escaped detection — may have reactivated after surgery and exaggerated the damaging immune reaction.
So for the new procedure, the medical team took extra precautions. Revivicor’s scientists developed new, more sensitive tests for viral DNA, and ran these on tissue samples taken from inside the animals, as opposed to just nasal swabs. They also tested the pigs for antibodies against cytomegalovirus, which could tell them if the animals had ever been infected and might thus still be harboring latent forms of the pathogen deep in their cells.
Both Bennett and now Faucette have received Revivicor’s experimental pig heart through the Food and Drug Administration’s expanded access, or compassionate use, pathway. Mohiuddin said a team of 30 FDA analysts looked at more than 400 pages of data from Bennett’s case as well as ongoing studies of non-human primates that the hospital submitted before giving the OK to proceed with a second patient. The longer-term aim is to move away from one-off experiments and into formal clinical trials.
In talks with the agency about starting such trials, Mohiuddin — who is best known for a pioneering 2016 study in which his team kept baboons with transplanted pig hearts alive for over a year with a unique cocktail of immunosuppressants — learned that, among other criteria, the FDA wants to see researchers consistently keep a large group of primates alive for at least six months post-transplant. His research team recently started a trial in primates with Revivicor’s 10-gene edited organ to try to prove they can do that.
“We have a few animals right now with beating hearts inside them,” he said in an interview with STAT. The longest is about three-months post-surgery and none of the animals have died, he said. If all goes well, he expects it to take at least a year or two to collect all the necessary data to satisfy the FDA. “But for patients like Mr. Faucette, they don’t have two years to wait. So we are presenting patients like this who may not have any other options available, at least we can give them a chance to live.”