
Sterile filling is a critical step in pharmaceutical primary packaging; the transfer of sterile drug product into its final primary container, such as vials or syringes, must be performed under controlled and aseptic conditions. However, during this process, some drug product loss is inevitable.
This is concerning for companies with high-value drugs whose active pharmaceutical ingredient (API) is costly, difficult to procure, or has lengthy manufacturing lead times. Furthermore, products for early-phase clinical evaluation require small batch filling, where losses are even more apparent and costly.
Sterile filtration and filling typically consume at least one liter of drug product on traditional aseptic filling lines. Minimizing drug product loss can improve yield and help to supply high-value drugs to clinical trials or for commercial use.
Sterile filtration
The greatest source of loss occurs during sterile filtration. During this step, the drug product is formulated in a large container and then passed through two sterilizing filters before it is transferred into a pre-sterilized vessel or bioprocess bag. Transfer losses, line losses, and filter hold-up are expected to occur during this process and contribute to product loss.
Transfer loss describes the solution remaining in the container that the product was formulated in and filtered from. 10-15 mL of drug product can be lost at this stage if care is not taken to empty the vessel fully.
Line loss refers to drug product left in the tubing. The primary source of this loss occurs between the two sterilizing filters. Filtration ends once the final portion of the drug product passes through the first sterilizing filter. Only pressurized air exceeding the bubble point of the initial filter can recover the drug product between these two filters. However, due to inherent risks involved, along with the need for special equipment and additional process steps, the product is typically discarded instead. Drug product remaining in the tubing after the filters can be expelled by manually compressing the line and working the solution out. Assuming most of this product is retrieved, a minimum of 15-20 mL of drug product will be lost to line losses.
Finally, sterilizing filters retain a significant amount of solution. A standard sterilizing filter that is used for approximately one liter of drug product will retain over 12 mL as hold-up volume. Since most filtration processes require two filters, at least 24 mL will be lost from filter hold up.
Strategies to reduce loss during sterile filtration
There are several approaches to limit drug product loss during sterile filtration. To reduce transfer loss, the drug product should be carefully siphoned from the formulation container to minimize the amount of product left behind. Line losses can be limited by reducing the length and inner diameter (ID) of tubing used in filtration. While reducing the length of the tubing will not affect filtration efficiency, narrowing the ID can extend filtration time. Therefore, it’s essential to choose an optimal tubing ID that limits loss without unnecessarily prolonging filtration.
Filter hold-up can be limited by performing filter studies to optimize the filter size and membrane. Smaller filters have less hold up, resulting in less product loss. The drug product may have a greater flux rate with certain filter membranes, allowing the selection of a smaller filter without worrying about clogging issues. This will help limit loss.
Filter hold-up can also be limited by reducing the number of filters used in sterile filtration. If it is possible to use one filter, it is something to consider; however, most fill processes will require two sterilizing filters.
Sterile filling
Product loss also occurs during the filling process. After filtration, the sterile drug product is connected to the filling line and filled into its primary container – such as a vial, syringe, or cartridge. During this process, product is lost to a line purge, line losses, transfer loss, and weight checks.
A line purge is the process of drawing drug product into the filling line and immediately dispensing a set volume of solution. This process purges the filling line of all air before the fill is started to ensure accurate and consistent volume dispensing. This typically results in a loss of at least 10 mL per filling line.
Throughout filling, weight checks are performed to ensure fill volumes are consistent across the entire batch. If destructive weight checks are performed, which is most common, then this check will consume one filled container every 50 to 150 filled units for each fill head used. In addition to this, several consecutive passing weight checks will be required to start the fill, and the final filled unit will be pulled for a weight check to bracket the end of a fill. The amount of loss depends on many factors – the fill volume, the frequency of weight checks, and how many fill heads are used – however, as an example, if weight checks are performed for every 100 units, the fill volume is 1 mL, and only one fill head is used, then at least 13 mL of drug product will be lost to weight checks for a 1,000 unit fill (that’s three initial weight checks, nine performed every 100 units filled, and one last weight check). More weight checks will be performed if any fall out of specification during the fill.
Finally, line losses and transfer loss are expected during filling. Air bubbles often get pulled into the fill line near the end of a fill. Air in the line signals that dispense volumes will not be accurate or consistent, and if these bubbles cannot be removed from the line before they reach the pump, then the fill will end.
Additionally, the fill will end once air reaches the outlet of the pump. Unlike solution, air is compressible. When air reaches the outlet of the pump, the air will compress during pumping, preventing the required volume of solution from moving through the fill line and being dispensed into the container. This can also cause dribble, as compressed air will return to its normal volume between pumps, pushing product out of the fill needle while the next container is being positioned for filling. Therefore, the fill ends once air is observed at the outlet of the pump. Any unused product remaining in the filling line will contribute to product loss.
Solutions to reduce loss during sterile filling
Reducing losses in sterile filling involves a few similar strategies to those used in filtration. Line losses can be limited by reducing the length and inner diameter (ID) of the tubing used. Reducing length will have no impact on the fill, but narrowing the ID of the tubing can add stress to the system and potentially slow filling. This should be optimized to ensure the fill progresses without too much backpressure in the filling line while limiting product loss.
Additionally, line loss and weight checks can be limited by opting for single-head filling. Each fill head requires its own fill line, weight checks, and line purge. Opting for one fill needle will significantly reduce loss from each of these areas. However, this approach will extend the duration of the fill. Using the fewest number of fill needles to complete a project within a reasonable timeframe will be useful to limiting product loss. Additionally, multi-head filling systems that draw solution from a shared manifold should be avoided if reducing drug product loss is a significant concern. This manifold pulls solution from a shared reservoir. This will all be lost at the end of the fill, contributing to greater overall product loss (see Figure 1).
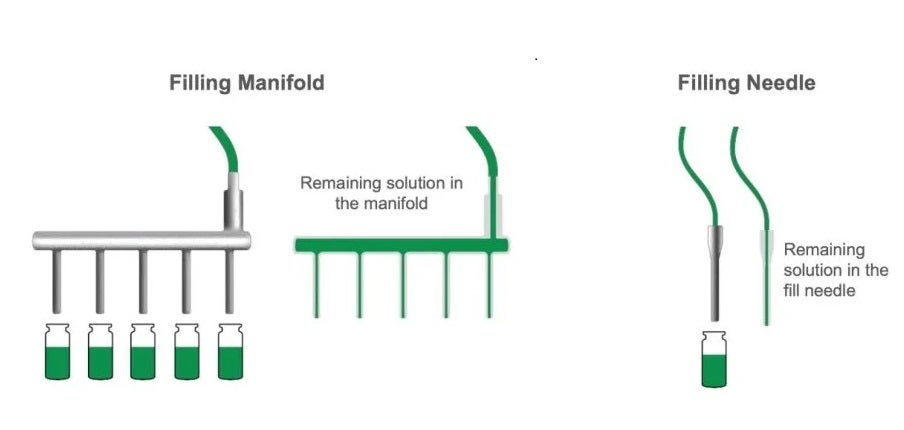
Figure 1. Filling Manifold (left) vs. Filling Needle (right). A filling manifold pulls solution from a shared reservoir which leads to greater line loss compared to a single fill needle.
Loss from weight checks can also be eliminated by opting for non-destructive weight check methods. If this is not an option, then the frequency of destructive weight checks may be able to be reduced (e.g. from every 100 units to every 150 units) to lower product loss. However, to do this, there must be confidence in the precision of dispense volumes.
The line purge may be able to be reduced as well. Typically, this volume is specified in the batch record and tends to be larger than necessary to ensure all air is purged from the filling line. If additional care is taken to eliminate air in the lines during the purge, it’s possible to decrease this volume.
To mitigate product loss caused by transfer losses, consider filling from a bioprocess bag (biobag) rather than a glass vessel. Biobags allow product to be drawn from the bottom of the bag, allowing the bag to be drained completely. In contrast, glass vessels siphon product through a dip tube, which must be completely submerged in solution to ensure air isn’t pulled into the filling line. This results in 100 mL or more of drug product remaining in the vessel at the end of a fill (see Figure 2). Using a biobag for filling, when feasible, helps reduce such losses.
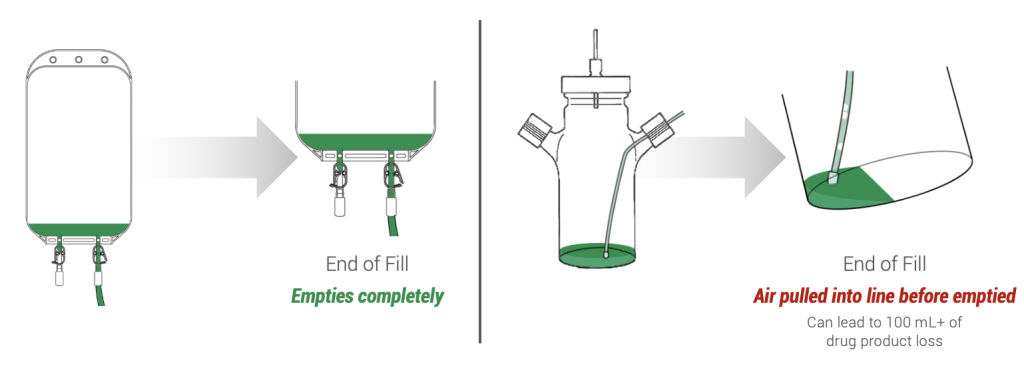
Figure 2. A spinner flask (right) vs. a biobag (left) at the end of a fill. Air bubbles will be caught in the fill line before all the solution is drained from the spinner flask, prematurely ending the fill. Biobags (left) will drain nearly all its contents before air is introduced into the fill line.
Finally, lifting the bulk drug product at the end of the fill can also cut transfer loss. This process encourages air that could be pulled into the fill line to rise back into the bag or vessel, preventing the air from being pulled towards the fill needle, which can lead to a premature end to filling activities due to inconsistent dispense volumes.
The total amount of drug product loss will depend on many factors, including the equipment used, the batch size, the drug product’s attributes, the filling line employed, the manufacturer, and much more. However, if all the above mitigation strategies are used, product loss could potentially be reduced to as low as 75 mL.
Introducing an innovative sterile filling method to further reduce product loss
While all the above actions can help mitigate product loss, their impact has limitations.
Berkshire Sterile Manufacturing (BSM), a subsidiary of Sharp providing aseptic fill finish services, developed and validated a new sterile filling process in 2023 aimed to reduce product loss to its minimal limit.
This new process has cut nearly all sources of product loss. The tubing used for filtration and filling spans just a few inches instead of the several feet that traditional methods require. The vessel used for filling is inverted and pressurized, offering a nearly complete transfer of solution, and weight checks are non-destructive. Additionally, a line purge is not needed.
BSM’s Low Loss Fill Process promises less than 30 mL of drug product loss for each filled batch, with losses often remaining between 5-15 mL. In this new process, BSM replaced a peristaltic or piston pump used in traditional filling lines with a time-over-pressure system to reduce line loss, transfer loss, and the line purge. The drug product is subjected to low pressure (typically 1 psi) and the dispense volume is controlled by a highly accurate actuator. There is no hand-pipetting involved, and the process is entirely isolator-based to offer the best sterility assurance.
Completing the process: secondary packaging
Following sterile filling, the injectable drug requires secondary packaging as the final step before it can be delivered to patients. After taking careful steps to minimize drug loss during sterile filling, it’s equally as important to minimize yield loss during secondary packaging activities.
High-value drug products require specialist handling and often need special storage conditions or packaging requirements, including cold chain storage, controlled light environments and custom kitting.
Conducting secondary packaging in close proximity to sterile filling will also reduce the complexity and risk associated with shipping drug product. Berkshire Sterile’s manufacturing site in Massachusetts is a four-hour drive from Sharp’s clinical and commercial packaging sites in Pennsylvania. This means drug product can be transferred from one site to the next within one business day, lowering the risk of damaged shipments, delays, or deviations from acceptable storage conditions.
The nuanced requirements of high-value drug products demanding specialized storage conditions and tailored packaging pose logistical challenges, especially for small batches. Finding a packaging and distribution partner capable of managing these unique requirements reduces complexity and minimizes risk for pharma clients.
About Sharp’s services
Together, Sharp and Berkshire Sterile Manufacturing specialize in fill/finish and secondary packaging of sterile injectables, with services to support vials, pre-filled syringes and cartridges/autoinjector and pen devices.
Offering a comprehensive array of solutions for sterile injectables, its experts have extensive experience managing the complexities of handling high-value biotech drugs.
In addition to packaging vial and pre-filled syringe formats, Sharp can also engineer and run a dedicated line for autoinjector and pen devices, including YpsoMate®, Molly® or Physioject™ autoinjectors. In fact, Sharp can label and package the majority of already available pre-assembled autoinjector and pen devices.
Finally, Sharp also offers design services, cold chain storage, packaging in temperature and light-controlled environments, kitting, serialization and specialty distribution – everything needed to ensure a safe, secure and reliable supply of your high-value drugs to market.
To learn more about how to optimize your packaging and distribution strategy, download our white paper below.

Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
