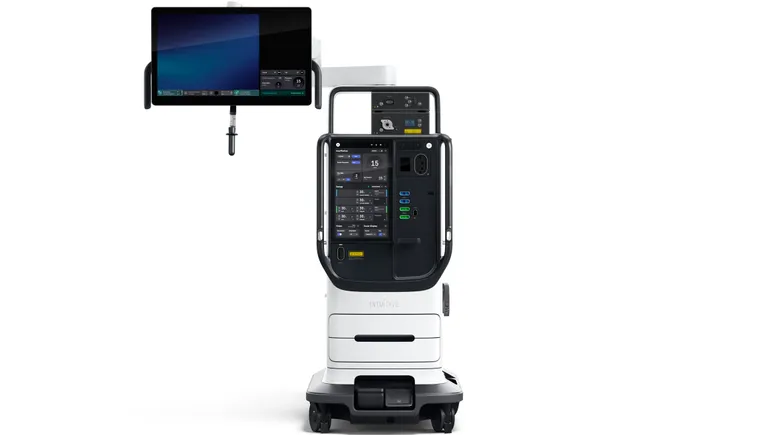
Intuitive Surgical has led the field of robotic soft tissue surgery for more than two decades, but deep-pocketed medical device makers and a host of smaller contenders are looking to muscle into the market.
To maintain its edge, Intuitive is rolling out a new version of its da Vinci robot in a limited launch this year and into 2025 with an array of upgrades and a price tag between 15% and 30% above its current model, depending on the options selected. The company secured U.S. regulatory clearance for its new da Vinci 5 robot last week.
Intuitive executives said on a Monday call with investors that the redesign, the robot’s first major overhaul in 10 years, integrates numerous improvements, some of which are expected to streamline the workflow of the operating room and shorten procedure times, ultimately lowering the cost of care.
That’s important, because research has shown that robot-assisted surgery produces treatment outcomes that are similar to conventional laparoscopic surgery but at higher costs.
“Faster cases that don’t compromise patient safety allow for more efficient use of precious human and capital resources at the hospital and should be well appreciated by our customers,” Intuitive Chief Medical Officer Myriam Curet said on the call.
The da Vinci 5 robot incorporates more than 150 enhancements in all, with many thought to be unique to the platform. For example, a feature called force feedback measures pressure exerted on body tissue using sensors built into the surgical instruments. The technology could help reduce tissue trauma.
“Intuitive’s [da Vinci 5] contains multiple features and advancements that, as best we currently know, are not included as part of Medtronic’s Hugo or [Johnson & Johnson’s] Ottava robotic systems,” Stifel analyst Rick Wise said in a note to clients.
More powerful computing capacity and a surgeon’s console that can be adjusted to help reduce strain on the physician during the operation are among the attributes of the new system that are expected to raise the competitive bar, Wise said.
The timing is right to incorporate upgrades such as the tissue-sensing haptic feedback, improved imaging and expanded computing power, because many of the technologies have come down in price in recent years, said Evercore ISI analyst Vijay Kumar.
“Certain ancillary technologies and costs have become more feasible,” Kumar said in an interview. “It certainly does help to have a competitive response when others are entering the field.”
The new platform also integrates multiple technologies, such as surgical cameras and energy generators, that healthcare systems now must acquire separately. Citi analyst Joanne Wuensch said, “Intuitive is moving closer to a one-stop shop with da Vinci 5.”
That strategy could affect several medtech companies that sell such procedure tools separately, said Stifel’s Wise.
“As hospitals adopt [da Vinci 5,] they may be less inclined to buy from other providers,” the analyst wrote.
Knocking at the door
Intuitive received Food and Drug Administration clearance for the first da Vinci system in July 2000, for use in laparoscopic surgical procedures. The company has dominated the market for robotic surgery ever since, with more than 10 million procedures performed using da Vinci, in areas ranging from hernia repair and prostate gland removal to bariatric and colorectal surgeries.
Medtronic and J&J, as well as smaller competitors such as CMR Surgical and Vicarious Surgical, are now looking to chip away at Intuitive’s lead.
Medtronic, which already sells its Hugo robot in international markets including Europe, Japan and Canada, could launch the system in the U.S. as soon as 2025.
“We expect Hugo, equipped with advanced digital capabilities, to be a meaningful growth driver for us in the years ahead,” Medtronic CEO Geoff Martha said last month on the company’s quarterly earnings call.
J&J expects to file a submission for an investigational device exemption in the second half of 2024 that will allow it to begin clinical trials for its Ottava robotic surgery platform. The Ottava robot is designed for use with J&J’s Ethicon surgical instruments, which J&J believes will generate interest in the system. But J.P. Morgan analysts have predicted it could take at least five years for J&J to gain FDA approval for Ottava.