Dive Brief:
- Johnson & Johnson has applied for premarket approval of its Varipulse pulsed field ablation (PFA) platform, the company said Monday.
- Biosense Webster, a subsidiary of J&J, filed for Food and Drug Administration approval using data from a study that found 80% of patients were free from atrial arrhythmia recurrence at one year.
- Boston Scientific and Medtronic are already approved to sell rival PFA devices in the U.S. J&J sees the integration of Varipulse with its Carto heart mapping system as a feature that sets it apart from the competition.
Dive Insight:
J&J has received clearances to sell Varipulse in the European Union and Japan this year. The next step is to win approval in the U.S., a market that is central to predictions that PFA will help add billions of dollars to atrial fibrillation (AFib) ablation sales in the coming years.
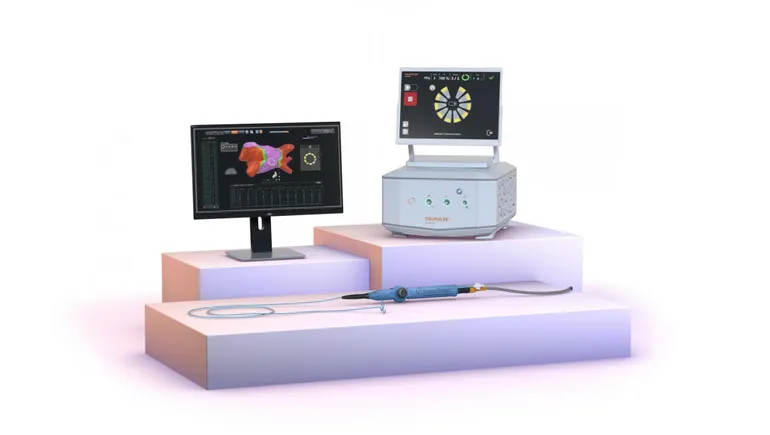
Varipulse is J&J’s initial play for the PFA market. The platform features a multi-electrode catheter, PFA generator and 3D mapping software. J&J CEO Joaquin Duato discussed the role the Carto software mapping plays in the company’s PFA strategy on an earnings call in January.
“[Carto] is a fundamental pillar of our strategy in cardiac ablation that supports procedural efficiency and, very importantly, now low to zero fluoroscopy workflow,” Duato said. “For the electrophysiologist, it’s very important to know where they are and what they are doing to the heart anatomy. Otherwise, they are flying blind if they don’t have a mapping system.”
Neither Boston Scientific’s Farapulse nor Medtronic’s Pulseselect have integrated mapping, although Medtronic received a CE mark for its Affera Mapping and Ablation System one year ago. Abbott has a mapping system, EnSite X, but is lagging the front three in the PFA race. The company started a clinical trial of its PFA device early this year, leading analysts to predict it could reach the U.S. market in 2026.
J&J has deployed 5,000 Carto systems globally, Duato said, and is developing a range of catheters to pair with the software. The idea is to enable electrophysiologists to choose the catheter that is right for each patient. Duato contends radiofrequency ablation, a separate ablation procedure, is “here to stay” despite the gaining momentum of PFA.
J&J’s electrophysiology unit reported nearly 25% year-over-year growth in the fourth quarter, with U.S. and ex-U.S. sales both increasing by more than 20%.