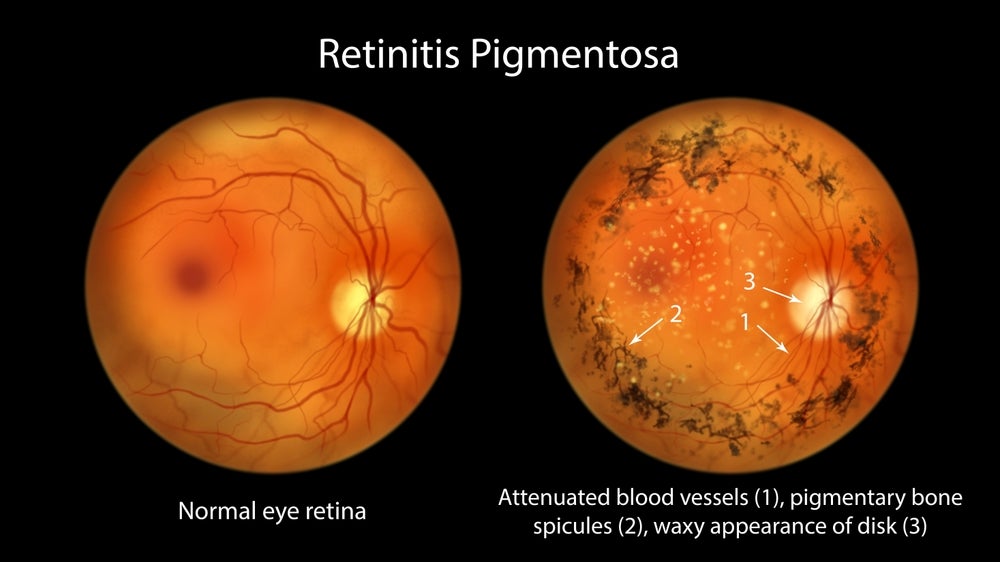
Nanoscope Therapeutics released promising top-line results for its RESTORE trial, which is studying the gene therapy MCO-010 in patients with retinitis pigmentosa (RP).
Currently, there is only one gene therapy on the market for retinitis, Spark Therapeutics’ Luxturna, which is a retinoid isomerohydrolase activator.
While MCO-010 will not be the first gene therapy to penetrate the RP market, it raises the prospect of introducing another mechanism of action into the retinitis space.
Additionally, MCO-010 is also in the pipeline for juvenile macular degeneration (Stargardt Disease), a group of inherited and rare retinal diseases for which there currently are no gene therapies available.
MCO-010, which has US Food and Drug Administration (FDA) Fast Track designation and FDA Orphan Drug Designation for both RP and Stargardt disease, is currently in Phase IIb development for RP in the RESTORE trial and is of paramount interest due to being a gene-agnostic optogenetic therapy.
Clinical trial results in patients with retinitis have shown that at 52 weeks, the high-dose and low-dose groups both exhibited statistically significant improvements in best-corrected visual acuity (BCVA) of >0.3 units on the LogMAR scale.
According to Nanoscope, the RESTORE trial is the first pivotal randomised controlled trial of a mutation-agnostic RP gene therapy that demonstrates a statistically significant improvement exceeding the clinically important BCVA >0.3 LogMAR threshold.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
The trial also met its key secondary endpoint, confirming that this improvement was continued in the high-dose group for 76 weeks, but not in the low-dose group.
Consequently, the high dose (1.2 x 1011 gc/eye) is the intended commercial dose for MCO-010.
Furthermore, the trial met another of its secondary endpoints: MCO-010 demonstrated improvements in the Y mobility test, which measures the patients’ abilities to navigate a room at varying luminance levels, and the shape discrimination test, at 52 weeks in both high and low-dose groups.
As for the safety profile of MCO-010, in general, the treatment was tolerated well and results were consistent with preceding trials.
Most adverse events were mild to moderate in severity, relating to anterior chamber cell and ocular hypertension.
No severe adverse events or serious treatment-related events were reported.
Additionally, there were no cases of intraocular inflammation in the treatment groups.
Further data from the RESTORE trial is set to be unveiled at the Annual Scientific Meeting of the Association for Research in Vision and Ophthalmology, which will take place in Seattle, Washington, US, on 6 May.
MCO-010, similar to Luxturna, is a gene therapy.
In spite of this, it stands out by offering an alternative mechanism of action.
MCO-010 is an optogenetic therapy that enables light-based vision restoration and reduces light-induced chronic retinal cell damage through delivering white opsin to target cells, which generates substantial photocurrent at white light intensity levels that resemble daylight conditions.
This has positive implications for patients, which include potentially introducing a second gene therapy into the RP space, as well as providing a treatment with a new mechanism of action for RP patients.
Key opinion leaders (KOLs) interviewed by GlobalData, a leading data and analytics company, noted that Nanoscope, in developing MCO-010, has shown some improvements, and is moving towards pivotal trials and that the therapy itself is interesting.
However, KOLs also pointed out that in general, a shared issue among optogenetic therapies is that it is unclear how high of a resolution patients can reach.
Nanoscope is planning to apply for FDA approval for MCO-010 in the second half (H2) of 2024 based on these Phase IIb results.
Should it receive approval, MCO-010 has tremendous potential to be popular among retinitis patients and clinicians alike, given its promising RESTORE trial results, and additionally the need for more therapies within the RP space.
