Dive Brief:
- Abbott said Tuesday it received the Food and Drug Administration’s approval to market a transcatheter device for repairing the tricuspid valve in patients who are unable to withstand open-heart surgery.
- The go-ahead from the FDA paves the way for Abbott’s Triclip repair system to compete in the U.S. against Edwards Lifesciences’ recently approved transcatheter tricuspid valve replacement device, Evoque.
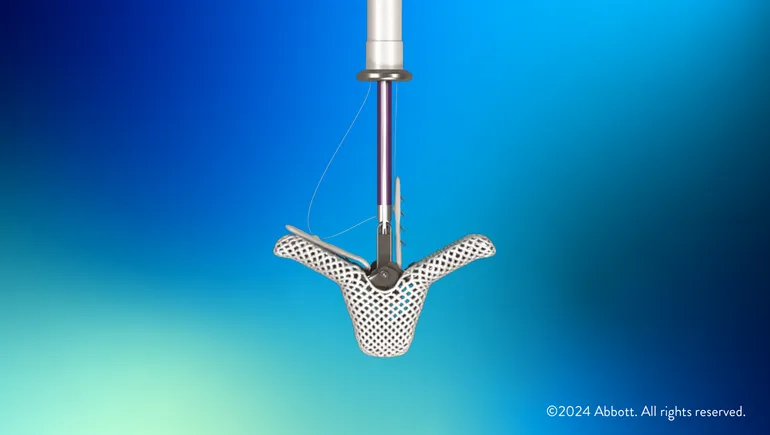
- Triclip uses the same clip-based technology to treat tricuspid regurgitation as Abbott’s Mitraclip for mitral valve regurgitation, a device the company has credited with driving double-digit growth in its structural heart business.
Dive Insight:
Valve regurgitation, or leaking, occurs when the heart valve does not close correctly as it opens and shuts to circulate blood. The tricuspid valve controls blood flow from the right atrium to the right ventricle. When the valve isn’t closing properly, blood can flow backward in the heart, forcing it to work harder.
Most common in older adults, the condition can increase the risk of heart failure, irregular heartbeat and blood clots.
Triclip builds on the technology used in Abbott’s Mitraclip device for mitral valve repair to help restore the performance of the patient’s own valve but is designed for the tricuspid valve’s anatomy. The device is delivered through a vein in the leg and works by clipping together part of the valve’s leaflets to help blood flow in the right direction.
The approval was supported by the company’s pivotal trial that compared the device to medical therapy in people with severe tricuspid regurgitation at intermediate or greater risk for open-heart surgery. The study found 90% of patients saw their condition reduced from severe or higher to moderate or less at 30 days, and the reduction was sustained at one year.
The Abbott repair device will compete against Edwards’ Evoque tricuspid valve replacement system, which gained FDA approval in February, becoming the first transcatheter treatment for tricuspid regurgitation in the U.S.
“We think this is a meaningful milestone for the transcatheter mitral and tricuspid therapies (TMTT) market as it gives [1.6 million] U.S. patients who cannot undergo surgery a viable treatment option,” RBC Capital Markets analyst Shagun Singh said Tuesday in a research note.
Meanwhile, BTIG analyst Marie Thibault expects Triclip to be “gradually adopted over time given the favorable pivotal data, unmet patient need and [Abbott’s] commercial footprint in structural heart solutions.” BTIG analysts estimate Triclip contributed about $100 million in revenue generated outside the U.S. in fiscal year 2023.
An FDA advisory panel convened in February found the benefits of Triclip outweighed the risks in a 13 to 1 vote.