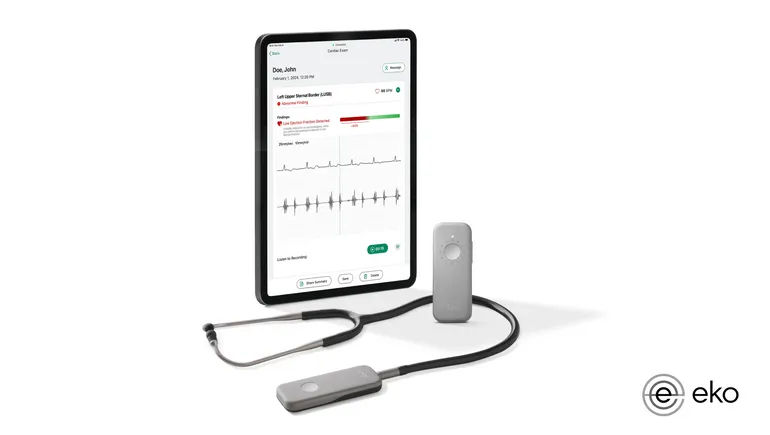
Dive Brief:
- Eko Health received 510(k) clearance for a stethoscope feature that can detect an indicator of heart failure in routine physical examinations, the company said Tuesday.
- The Food and Drug Administration clearance covers artificial intelligence that expands the capabilities of Eko’s Sensora Cardiac Early Detection Platform for the detection of low ejection fraction (EF), when the heart pumps too little blood from the left ventricle.
- Eko sees the tool improving the detection of heart failure by making the identification of low EF in 15 seconds part of routine stethoscope exams performed in primary care settings.
Dive Insight:
Primary care settings often lack access to echocardiography and other traditional heart failure detection tools because they are expensive, require specialized training and add significant time, Eko said. Adding low EF detection capabilities to digital stethoscopes could remove those barriers to the detection of heart failure and lead to more patients getting diagnosed before they develop severe symptoms.
Eko developed the AI tool in collaboration with Mayo Clinic.
“The ability to identify a hidden, potentially life-threatening heart condition using a tool that primary care and subspecialist clinicians are familiar with – the stethoscope – can help us prevent hospitalizations and adverse events,” said Paul Friedman, chair of the department of cardiovascular medicine at Mayo Clinic, in a statement from Eko. “Importantly, since a stethoscope is small and portable, this technology can be used in urban and remote locations, and hopefully help address care in underserved areas.”
Eko received FDA breakthrough designation for a low EF screening algorithm in 2019. The FDA granted the regulatory privileges to Eko after the researchers showed the model had sensitivity, specificity and accuracy of around 86% in a study of more than 50,000 patients.
The agency cleared Eko algorithms intended to help spot atrial fibrillation and heart murmurs in 2020.
In 2023, Eko launched its Sensora platform and discussed its work on two new machine learning algorithms for analyzing electrocardiogram and heart sound data. One of the algorithms was for low EF. The other AI was designed to detect and stratify pulmonary hypertension.
Eko received a $2.7 million grant to support its work on pulmonary hypertension in 2022.
The low EF clearance is the latest in a series of FDA authorizations of AI-powered devices. This year, the agency has authorized software for detecting a lung condition, assessing dementia risk, spotting skin cancer and cervical cancer screening.