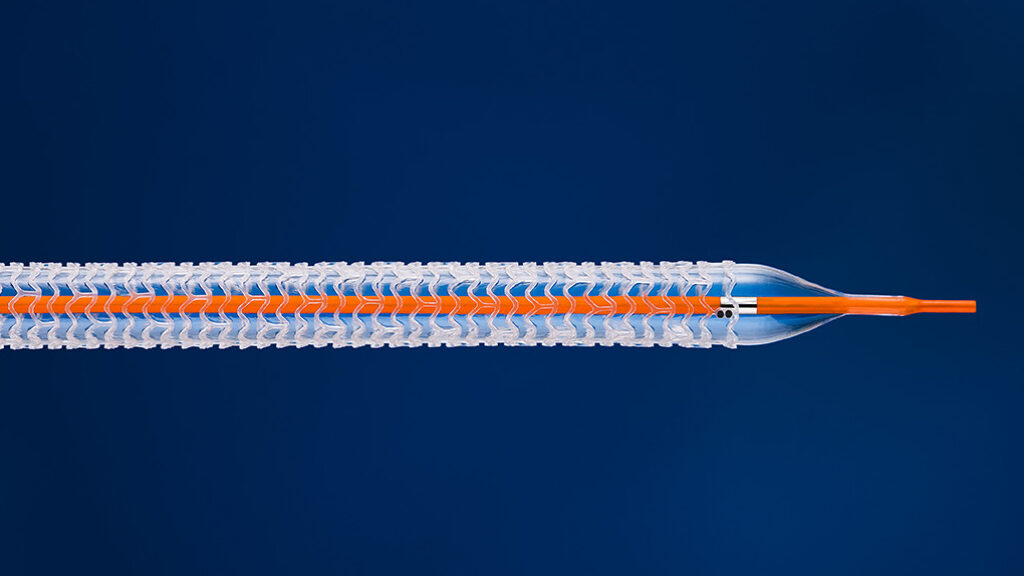
An Abbott device that failed in heart disease patients is getting a new life in patients with severe vascular disease.
The device is a below-knee stent that widens clogged blood vessels, and then vanishes into the vessel’s walls over the course of three years. It also delivers a drug that prevents scar tissue from forming — a common risk factor with traditional metal stents that further narrow the vessel.
advertisement
Abbott’s first dissolvable stent, for coronary artery disease, received Food and Drug Administration approval in 2016. But the company voluntarily pulled the product from the market just over a year later due to “low commercial uptake.” The device also received mixed results in clinical trials.
STAT+ Exclusive Story
Already have an account? Log in

Get unlimited access to award-winning journalism and exclusive events.