Dive Brief:
- Disruption to the supply of BD Bactec blood culture media bottles is expected to impact patient diagnosis and follow-up patient management, the Food and Drug Administration said Wednesday.
- BD told customers in June they may experience “intermittent delays” in supply over the coming months. The company said reduced availability of plastic bottles from its supplier had stopped it from manufacturing Bactec to meet full global demand.
- The FDA is advising laboratories to “develop strategies to prioritize the use of blood culture media bottles, based on clinical need, to maintain quality and safety of patient care.” On Wednesday, the agency added blood culture media bottles to the medical device shortages list.
Dive Insight:
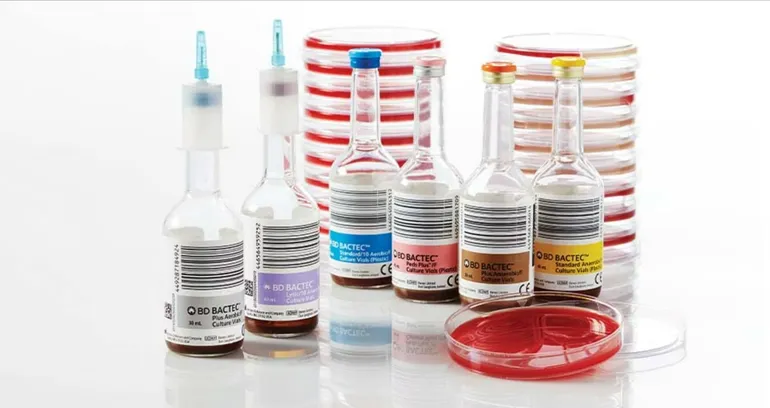
Laboratories use BD Bactec bottles to detect pathogens in blood taken from children and adults. BD sells a range of bottles containing different media for detecting aerobic or anaerobic organisms. Healthcare professionals choose what type of organism they want to check for, add blood to the bottle and send the mixture for processing.
BD sources the bottles from a supplier. By June, the company said it had been contending with reduced availability of the plastic bottles for several months. BD had managed the constraints “with a variety of measures,” the company told customers, but said users may start to experience delays in availability.
Initially, BD expected reduced availability to be temporary. However, the company said its investigation found “the issues are more complex than the supplier originally communicated.”
One month after BD issued its initial warning, the FDA sent a letter to healthcare providers about disruption to the availability of the bottles. The agency is advising users to perform blood culture collections “when medically necessary” and to prioritize the use of the vials for patients with clinical signs and symptoms of a bloodstream infection. The recommendations are intended to help users preserve supply for patients at highest risk.
BD said it expects inventory to “tighten in the coming weeks” before ongoing mitigation efforts “lead to increased supply to meet global demand.” Until then, the company said it will “fill customer orders regularly and as supply is available.” BD plans to share a supply update by September.
The company said in a Wednesday statement that it does not expect the supplier issue to have a material financial impact on its business.