Dive Brief:
- Truvian Health has raised $74 million to support a filing for clearance of its benchtop blood test instrument, the company said Wednesday.
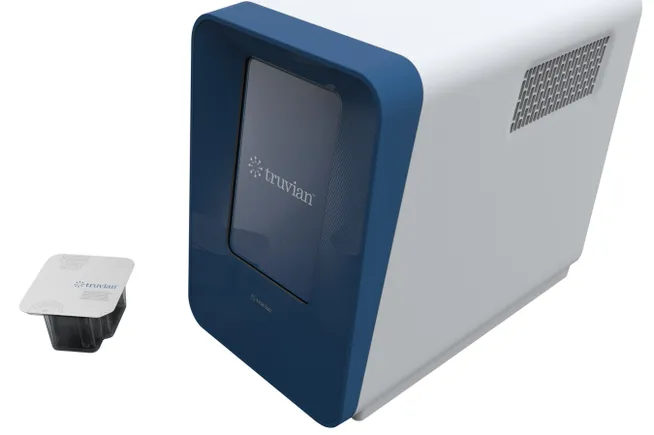
- The device is designed to perform routine blood tests, freeing clinics, doctor’s offices and pharmacies from the need to send samples to central labs.
- Truvian has shared research on its platform in a preprint study and in posters at medical conferences.
Dive Insight:
Truvian has identified reliance on central labs for processing blood samples as a barrier to the timely and cost-effective diagnosis and monitoring of health conditions. Healthcare providers can need to take large blood draws, which some physician’s offices are unable to perform on site, to collect samples for the analyzers that central labs use to run assays. It can take days to receive results and errors are possible.
Analyzing samples where they are collected could mitigate some of the risks, and performing assays on smaller volumes of blood would simplify collection. Truvian is pitching its platform as a product that can achieve those improvements.
The device works with blood samples collected from capillaries and veins. Users load the consumables and sample into the device, run the assay panel and receive results in 30 minutes. In the preprint study, Truvian ran 32 routine blood tests including immunoassay, clinical chemistry and hematology assays on 300-microliter samples. A single drop of blood is around 35 microliters.
The preprint study, which has not been peer reviewed, found Truvian’s platform had a run reliability of more than 95%, regardless of whether the instrument was used at its headquarters or a clinical trial site. Run reliability is the percentage of test runs that generate results successfully.
Truvian compared the results to data on samples analyzed using instruments including Roche’s Cobas devices to assess concordance with central lab results. The company found concordant results “for the majority of tests with no observed differences between internally and externally collected data.” Truvian has updated its device since running the study, including by reducing the turnaround time to 30 minutes.
Wittington Ventures and Great Point Ventures are supporting Truvian’s work to bring the instrument to the U.S. market. The investors co-led the financing round. Truvian also revealed Shoppers Drug Mart, Canada’s largest pharmacy, has joined as a commercial partner.