Dive Brief:
- Procept Biorobotics received Food and Drug Administration approval to start a pivotal trial of its robotic-assisted aquablation therapy in prostate cancer.
- The trial will compare the therapy, which is already used in noncancerous prostate enlargement, to radical prostatectomy in people with localized prostate cancer that is likely to grow at a slow to moderate rate, according to the Monday announcement. Truist Securities analysts said the trial could unlock a $500 million market.
- Procept has received FDA breakthrough designation in prostate cancer. With the trial having a six-month primary endpoint, William Blair analysts said Procept could win approval in 2026.
Dive Insight:
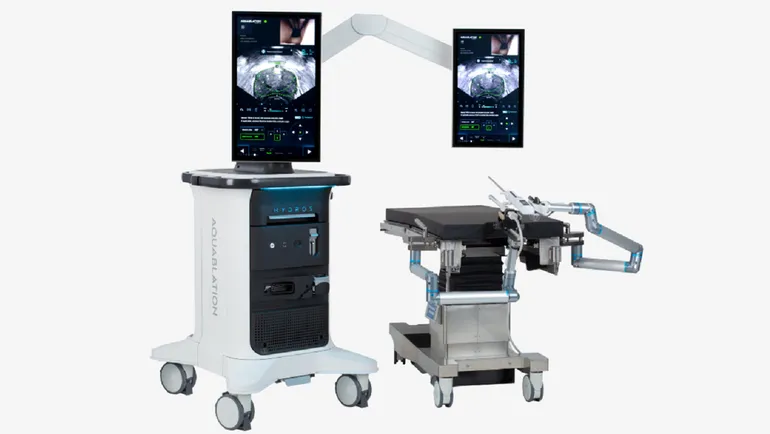
Procept’s surgical robot delivers a pressurized jet of fluid to cut and remove prostate tissue. The device is authorized for use in men with lower urinary tract symptoms caused by benign prostatic hyperplasia, a setting in which Procept received 510(k) clearance for an updated design in August.
The newly FDA-approved trial will test the approach in the treatment of some forms of prostate cancer.
Radical prostatectomy is the removal of the entire prostate gland. Procept describes aquablation therapy as a way for surgeons to personalize treatment according to a patient’s anatomy and “specify which areas of the prostate to remove while preserving the anatomy that controls erectile function, ejaculatory function and continence.”
Procept will enroll up to 280 men with Grade Group 1 to 3 localized prostate cancer and evaluate a co-primary endpoint based on morbidity after six months. Investigators will track patients for 10 years to assess the rate of treatment-related harm and oncologic events associated with aquablation and prostate removal.
William Blair analysts said in a Tuesday note to investors that 80% of radical prostatectomies are performed using Intuitive Surgical’s da Vinci robots.
However, Truist analysts, after talking to Procept executives, said Procept’s aim is to expand the market by treating “watchful waiting” patients rather than replacing da Vinci. Today, some patients do not receive treatment for prostate cancer. Rather, they are choosing to have their condition monitored to spare themselves the adverse effects seen in some people who have their prostates removed, Truist analysts said.
Meanwhile, Leerink Partners analysts wrote in a recent note to investors that cryotherapy and high-intensity focused ultrasound are existing options for the more than 2 million U.S. patients with low- to intermediate-risk prostate cancer.
Procept’s trial will study whether aquablation can treat prostate cancer without exposing patients to the adverse effects of radical prostatectomy.
Leerink analysts said the trial’s co-primary endpoints will look at the risk of incontinence and erectile dysfunction after six months. Existing clinical data show 20% to 55% of men have erectile dysfunction after prostate removal, William Blair analysts said, and around 20% of patients have urinary incontinence. Studies of aquablation have reported “low-single-digit to 0% rates,” the analysts wrote.
A trial comparing aquablation to prostatectomy for benign prostate treatment found Procept’s procedure was associated with shorter hospital stays.