What You Should Know:
– PathAI, a provider of AI-powered pathology solutions, today announced the launch of PathAssist Derm, an innovative tool designed to streamline the analysis of skin lesions.
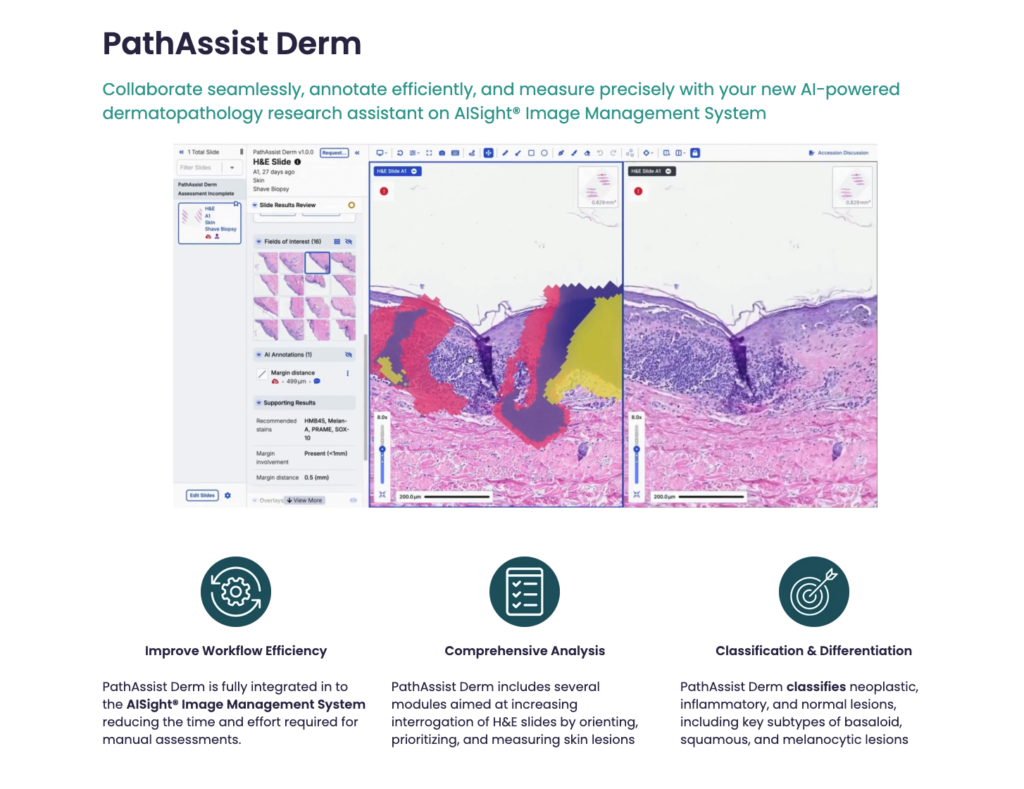
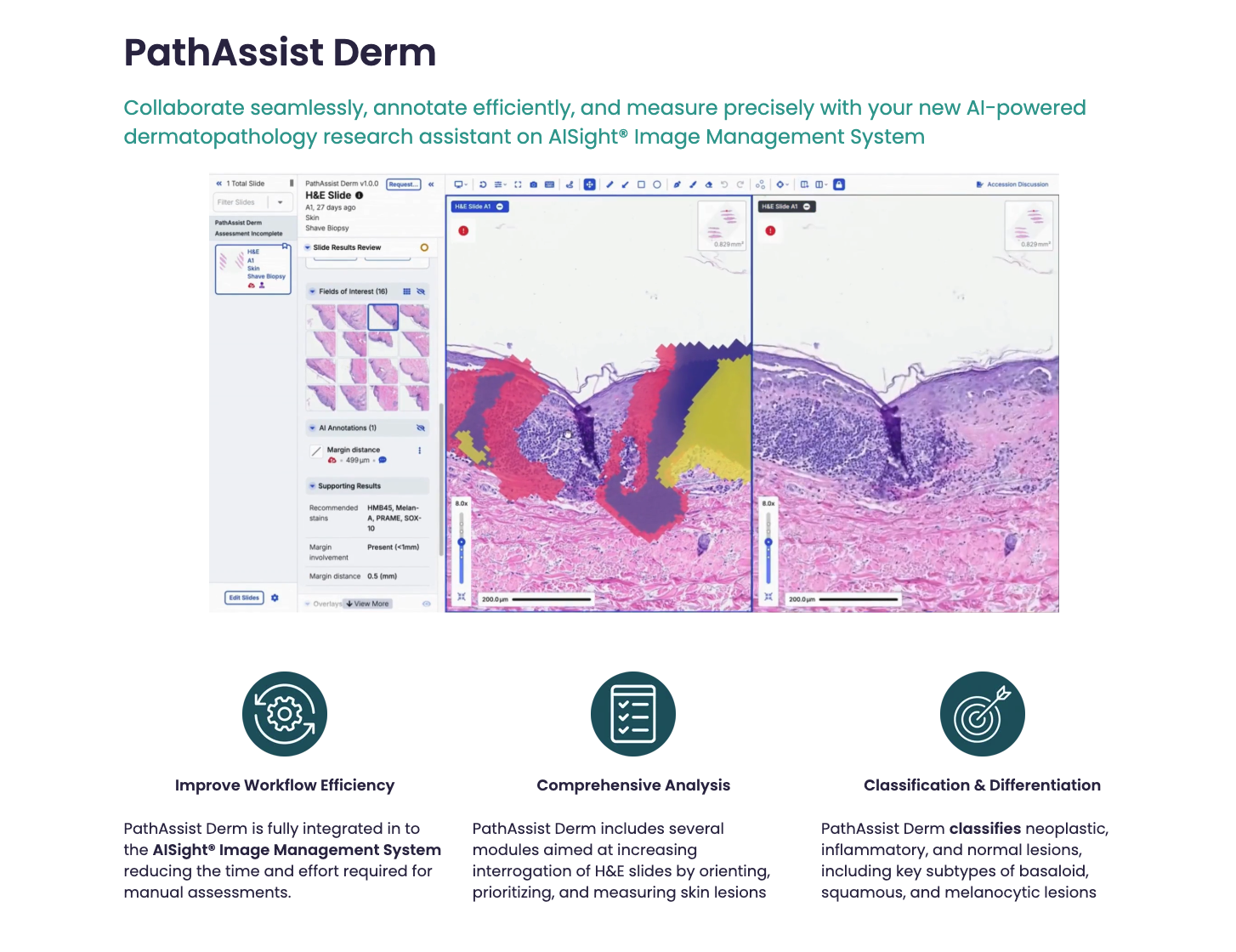
– Available now on the AISight® Image Management System (IMS), this research tool automates specimen orientation and identification, enabling pathologists to review samples more efficiently and accurately.
PathAssist Derm Capabilities
PathAssist Derm is the latest addition to PathAI’s suite of AI-powered solutions available on the AISight® IMS. This comprehensive platform provides pathologists with a range of tools to streamline their workflows and improve diagnostic accuracy. PathAssist Derm offers several key capabilities:
- Automated Specimen Orientation: Eliminates the need for manual orientation of glass slides, saving pathologists valuable time and reducing the risk of errors.
- Lesion Identification: Predicts the presence of 17 different skin lesion entities, including common and rare conditions, aiding in diagnosis and research.
- Detailed Measurements: Provides precise measurements of lesion size and characteristics, facilitating quantitative analysis.
“Skin cancer remains the most prevalent cancer worldwide, with over 1.5 million new cases diagnosed annually, which are associated with 60,000 deaths from melanoma alone,” said Andrew Beck, Co-Founder & CEO of PathAI. “These statistics present a critical opportunity for innovation in skin cancer research, both in the diagnostic and therapeutic realms. Our goal with PathAssist Derm is to provide researchers with a scalable tool that enhances dermatopathology research through AI-enabled lesion characterization and workflow efficiency, as part of our overall vision to improve patient outcomes with AI-powered pathology.”
PathAI will be presenting performance results for PathAssist Derm at the upcoming USCAP meeting in March 2025 and is actively partnering with laboratories to further investigate the tool’s impact on efficiency and diagnostic accuracy.