
The biomanufacturing sector is undergoing a revolution. Groundbreaking therapies are opening up new frontiers in the treatment of intractable ailments including cancer, heart disease, diabetes and haemophilia. As these therapies advance through preclinical and clinical trials, focus on stringent quality controls has never been greater.
Monoclonal antibodies (mAbs), cell and gene therapies (CGTs) and biosimilars are at the forefront of this transformation. Each could generate billions of dollars of value across the pharmaceutical sector – and bring with them intensified regulatory scrutiny for their creators and sponsors. A critical component of transforming their potential into reality is detection and elimination of host cell proteins (HCPs). Contamination from these proteins can compromise the quality, safety, and efficacy of biologics therapies.
Biomanufacturers are turning to HCP analytics services to mitigate the risks. Against the backdrop of a dynamic and rapidly evolving landscape, understanding the services on offer, and how they can help to shore up quality, is more important than ever.
The rise of importance of HCP analytics
HCPs were not always regarded as a biomanufacturing bugbear. Many are benign; identifying and eliminating them has hitherto been an afterthought in drug development. But recent research has cast them in a new light, with some posing significant risks due to their immunogenicity and unfavourable interactions with drug substances. Proteases and lipases, for instance, can directly degrade the drug or formulation buffer components and reduce effective product dosages.
Consequentially, regulatory activity around HCPs is ramping up. Guidelines from the International Conference of Harmonization (ICH Q11) and the US Food and Drug Administration (21CFR610.13) require biopharmaceutical developers to report HCP levels within strictly designated ranges. The US FDA mandates that HCPs must exist at levels below 100 ppm in formulated products.
ELISAs have been the standard method for HCP analysis. However, even the most sensitive ELISAs are limited by the quality of their antibodies, which are dependent on the antigens used for their generation and technical know-how and skills of the HCP antibody developer. And ELISAs provide only a total HCP count, without detailing individual HCPs that might be problematic. To address this, advanced orthogonal methods such as antibody affinity extraction (AAE) and mass spectrometry (MS) have evolved. AAE, for example, uses a broadly reactive HCP ELISA antibody to extract and concentrate downstream HCPs from a drug substance. MS can then facilitate more precise identification of individual HCPs.
Implementing advanced HCP analytics early in process development can provide significant advantages. As well as enhancing safety and efficacy, minimizing the need for later-stage process changes helps firms to crack down on development costs. And the market is brimming with expert service companies offering tailored options that allow these specialized analytical tasks to be outsourced.
The HCP analysis services you need to know about
Ensuring the safety and efficacy of novel biological drugs is a clear priority for biomanufacturers and regulators. Knowing what kind of analytics services are on offer, and how they help to guarantee this safety and efficacy, is therefore vital for stakeholders across the pharmaceutical industry.
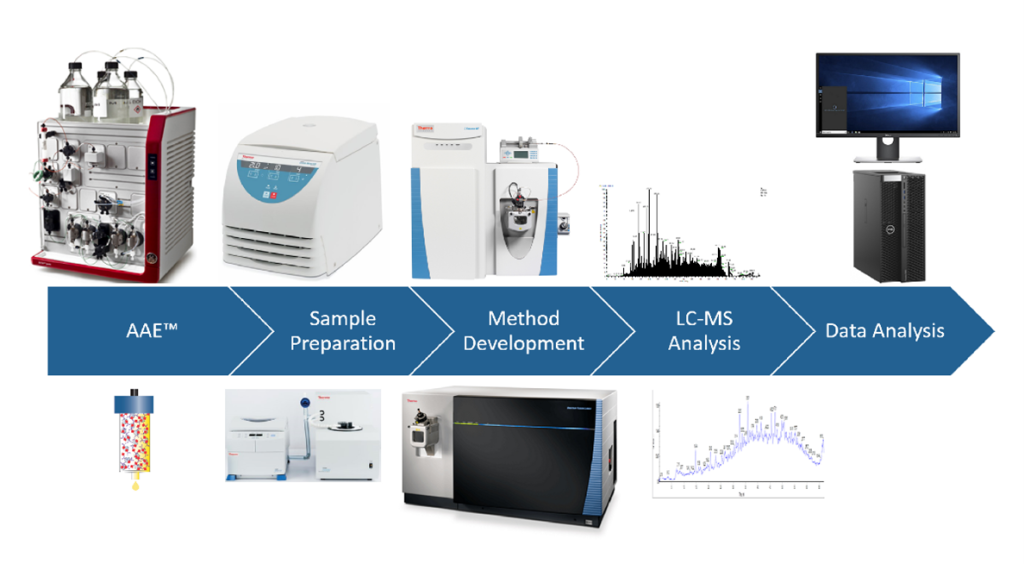
Four deserve particular attention. First, custom antibody production and assay development. Leading HCP analytics service providers will work with clients to develop tailor-made kits meeting specific process monitoring, release testing and regulatory needs. Cygnus Technologies, for example, has created ELISAs for over 25 different recombinant expression systems, spanning bacteria, yeast, fungi, human and mammalian cell lines, as well as transgenic and plant expression systems. In addition, their advanced Mix-N-Go™ platform offers a single-step sample treatment procedure and simplifies residual Protein A and Protein L immunoassays. And they offer state-of-the-art orthogonal methods including AAE and MS to guarantee a therapy’s safety.
Second, advanced antibody development. By generating sensitive, broadly reactive antibodies, HCP analytics providers can target host cell proteins from diverse expression systems, ensuring comprehensive coverage. Cygnus’s approach to antibody development sees the company offering both bulk and affinity purification of antisera – helping to obtain both crude IgG fractions or highly specific antibody activity, yielding unbeatable antibody purification and characterization services. And once again the company’s AAE method helps to refine a manufacturer’s anti-HCP antibody coverage analysis.
Turning to regulatory compliance, assay qualification needs to meet standard FDA, EMA and ICH guidelines. HCP analytics providers have a range of tools to help with this, including dilution linearity studies, spike and recovery analysis, interference and precision studies. Deploying orthogonal methods, such as AAE for antibody coverage analysis and MS identification of individual host cell proteins, is vital; Cygnus has pioneered these techniques to maximise their predictive performance in HCP ELISAs, facilitating the identification of individual downstream HCPs and bolstering MS sensitivity.
Sample testing is the final piece of the puzzle in ensuring biopharmaceutical products meet stringent quality standards. Cygnus provides extensive sample testing services using ELISA, AAE, and MS to analyze assay dilution linearity, spike recovery, and anti-HCP antibody coverage. This testing identifies downstream HCPs that may co-purify with the product, helping manufacturers to weed them out and cement their product’s fitness for purpose. MS, combined with AAE, allows for the precise identification of individual HCPs, guiding purification process development and validating the final drug substance. This can shed light on potential HCP issues early on in process development, saving biomanufacturers time, money and resources.
Custom antibody production, advanced antibody and assay development, assay qualification, sample testing: all have their place in helping biomanufacturers meet new regulatory demands, optimize purification processes and safely deliver therapies to patients. While navigating clinical pathways may seem tricky, working with an experienced HCP analytics service provider can make the journey much more straightforward. Cygnus is one such provider; with decades of experience in assessing and eliminating bioprocessing impurities, they are already helping trailblazing biomanufacturers expedite the road to market, and are on hand to help you too. Download the whitepaper on this page to find out more.

Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
