What You Should Know:
– Sonio, a medtech company dedicated to women’s and children’s health, announced today that it has received CE marking for its innovative product, Sonio Detect.
– The AI-powered software, already available in the United States, is now poised to enhance ultrasound examinations and improve care quality in France and throughout Europe.
Sonio’s Mission and Milestones
Founded in 2020 with the mission to improve the health of women and children globally, Sonio specializes in developing cutting-edge AI solutions for obstetric and gynecological ultrasound. The company has achieved several significant milestones, including receiving FDA 510(k) regulatory clearance for Sonio Detect in July 2023 and securing its first U.S. customer, Pediatrix Medical Group, Inc., in February 2024. With the CE mark for Sonio Detect, the company is now ready to broaden its reach and impact in the European market.
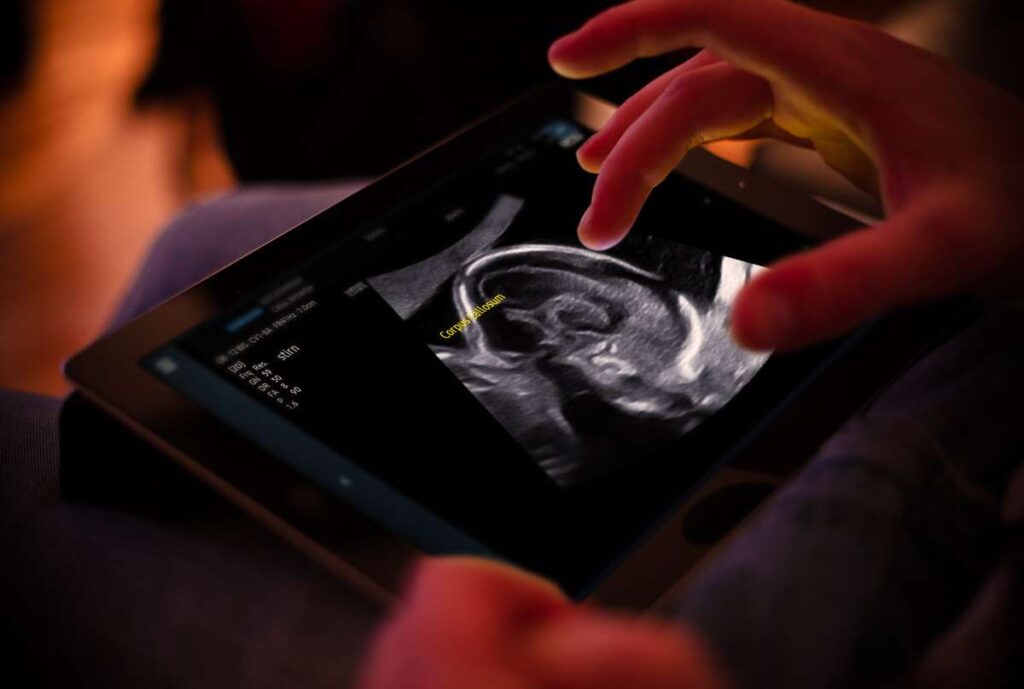
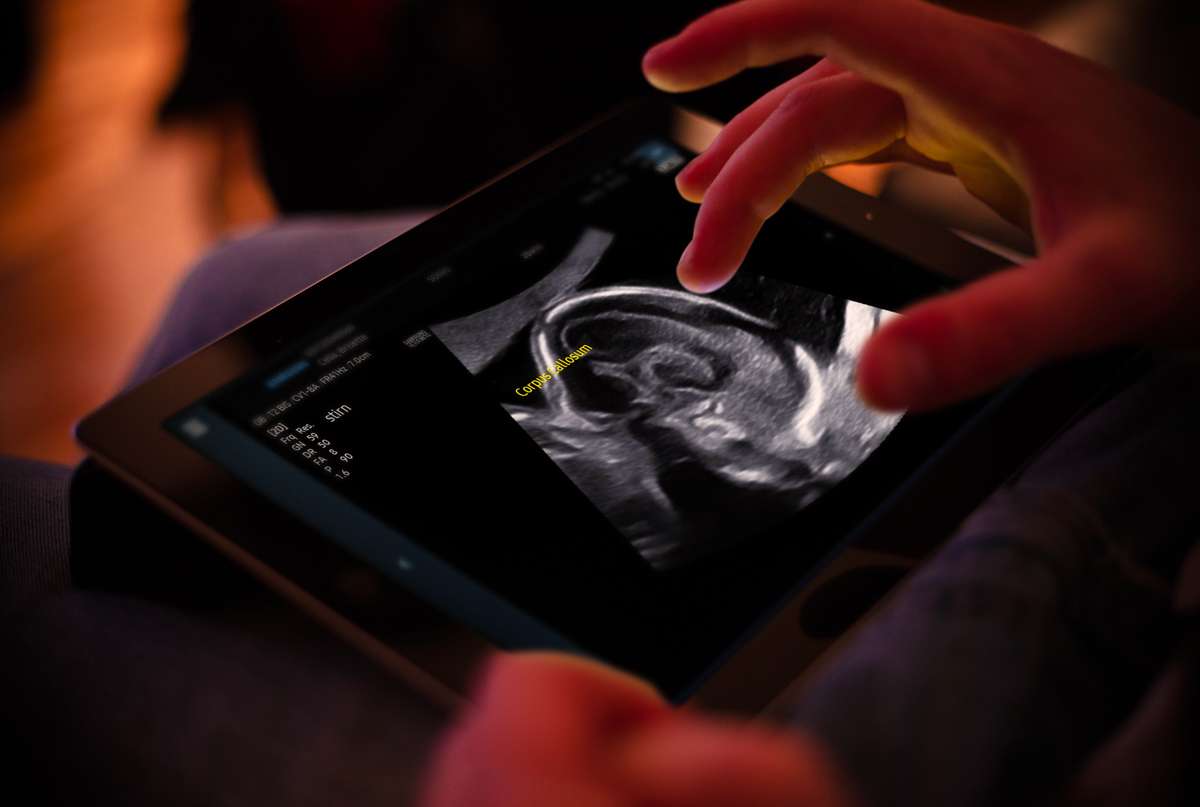
Sonio Detect: Ensuring High-Quality Ultrasound Examinations
Sonio Detect is designed to elevate the standard of ultrasound examinations by automatically detecting views, anatomical structures, and predefined quality criteria in ultrasound images. This cloud-based ultrasound reporting software guarantees continuous updates, incorporating the latest advancements in imaging technology, AI algorithms, and clinical decision support systems. Sonio’s commitment to innovation empowers healthcare providers to improve screening accuracy and enhance patient care.