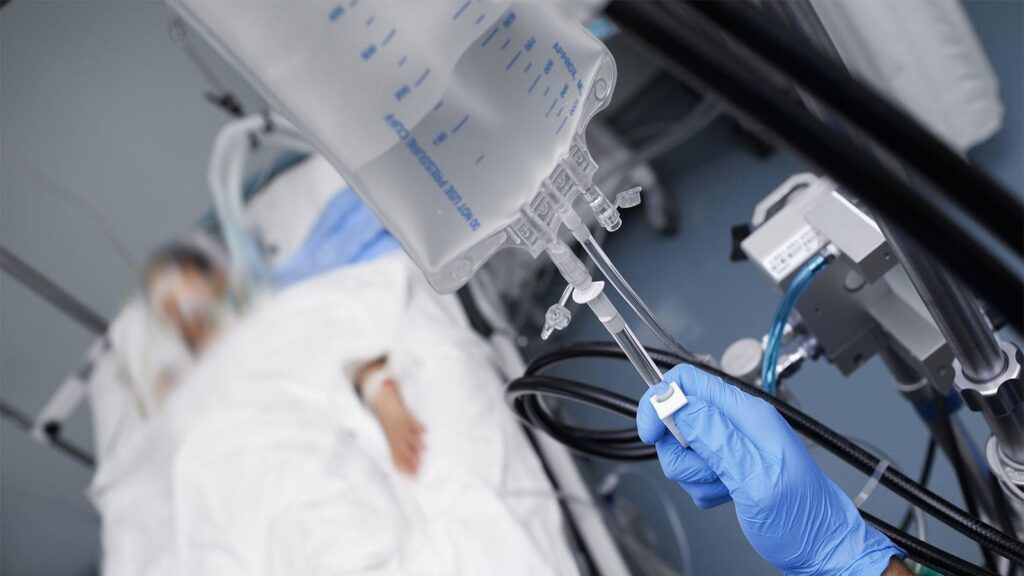
A Dallas anesthesiologist was sentenced Wednesday to 190 years in prison for injecting a nerve-blocking agent and other drugs into bags of intravenous fluid at a surgical center where he worked, leading to the death of a coworker and causing cardiac emergencies for several patients.
The emergencies began 2 days after Raynaldo Rivera Ortiz Jr., MD, was notified of a disciplinary inquiry into an incident during which he allegedly “deviated from the standard of care” during an anesthesia procedure when a patient experienced a medical emergency.
Ortiz, who had a history of disciplinary actions against him, complained to other physicians that the center was trying to “crucify” him.
Court documents show that Ortiz, who was arrested in September 2022 and convicted in April, waived his appearance at sentencing in federal court.
An attorney listed in court documents for Ortiz did not immediately return a phone call for comment.
Prosecutors said numerous patients at Surgicare North Dallas suffered cardiac emergencies during routine medical procedures performed by various doctors from May through August in 2022. Another anesthesiologist who had worked there died while treating herself for dehydration using an IV bag from the facility, prosecutors said.
The surgical center staff concluded that these cases suggested a pattern of intentional adulteration of IV bags used at the center.
They identified 10 additional unexpected cardiac emergencies that occurred during otherwise unremarkable surgeries in the months before his arrest, which was an exceptionally high rate of complications over such a short period, according to the complaint.
His medical license was suspended following his arrest by the Texas Medical Board.
Please enable JavaScript to view the