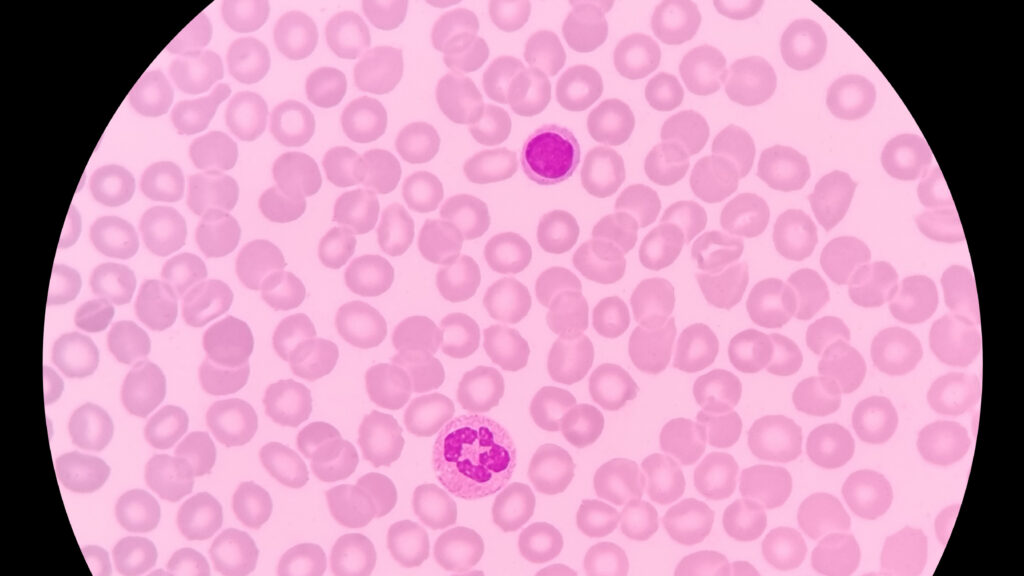
Belgium drugmaker Argenx said Tuesday that its antibody medicine failed to achieve the goals of a late-stage study for a platelet-destroying autoimmune disorder — a setback in the company’s efforts to expand the drug’s use.
Argenx shares were down 12% in early trading.
advertisement
The negative study, called ADVANCE-SC, was the second of two clinical trials involving the Argenx drug called efgartigimod in patients with primary immune thrombocytopenia (ITP), a debilitating condition that destroys blood-clotting platelets. When severe and uncontrolled, ITP can force patients to have their spleen removed.
Get unlimited access to award-winning journalism and exclusive events.