This story first appeared in The Readout newsletter. Sign up for The Readout and receive STAT’s award-winning biotech news delivered straight to your inbox.
Good morning, major news this morning, so let’s get straight to it.
advertisement
Trump’s win paves way for MAHA movement, FDA shakeup
Donald Trump’s election victory marks a new era for the biopharma industry. He campaigned on promises to shake up public health institutions, reshape federal health programs, and slash high costs across the system. He’s also said he’s ready for campaign lieutenants like Robert F. Kennedy Jr. to “go wild” on health, medicine, and food policy.
Trump repeated that promise in his victory speech. “We can add a few names like Robert F. Kennedy Jr.,” Trump told his supporters. “And he’s going to help make America healthy again.… He’s a great guy and he really means that he wants to do some things, and we’re going to let him go to it.”
Trump will have a Republican Senate to help implement his agenda and confirm his nominees, as the GOP picked up the required seats to take back the Senate majority. The Associated Press has not yet called which party will control the House of Representatives.
advertisement
Read more from STAT’s Sarah Owermohle.
Read STAT’s Lizzy Lawrence on how Trump’s embrace of Kennedy might translate into real policy.
And here’s STAT’s Matt Herper on what Trump’s victory might mean for the FDA specifically.
Novo posts strong Wegovy sales, sending shares up
Here are the highlights from Novo Nordisk’s third quarter earnings report this morning: Quarterly profit reached 33.8 billion Danish kroner ($4.88 billion), compared to forecasts of 33.5 billion kroner, according to consensus data compiled by Visible Alpha. Sales of Wegovy reached 17.3 billion kroner ($2.5 billion), versus expectations of 15.8 billion kroner. Overall, the obesity care business was up 44%.
This will be seen as a win for Novo, particularly after its competitor Eli Lilly reported earnings last week that fell below forecasts. Novo shares were up more in early trading.
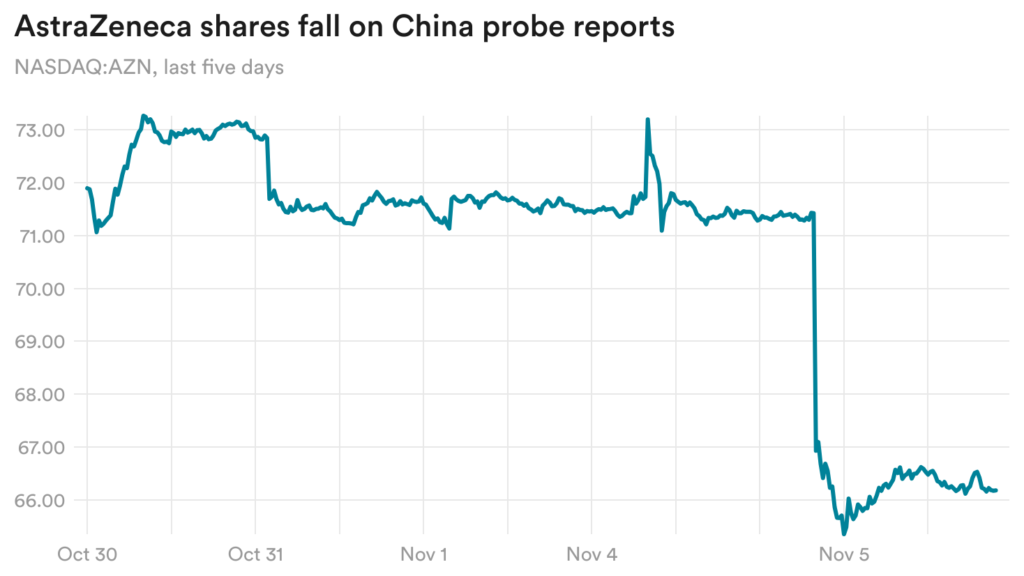
AstraZeneca falls on reports of Chinese probe
Shares of AstraZeneca dropped more than 7% yesterday after the Chinese publication Yicai Global reported that dozens of senior executives in the company’s China subsidiary have been implicated in an insurance fraud case.
This comes after the company last week disclosed that Leon Wang, the president of the China subsidiary, was under investigation by Chinese authorities.
In a statement, AstraZeneca said, “as a matter of policy, we do not comment on speculative media reports including those related to ongoing investigations in China. If requested, we will fully cooperate with the Chinese authorities. We continue to deliver our life changing medicines to patients in China and our operations are ongoing.”
.png)
As STAT contributor Brian Yang reported earlier this week, the probe of Wang raises questions about the company’s tactics in China. There’s been speculation that it’s connected to allegations that local employees were altering genetic testing results of patients so that China’s medical reimbursement agency would cover use of the cancer therapy Tagrisso.
Arcellx, Gilead post promising CAR-T safety data
An experimental CAR-T therapy for multiple myeloma from Arcellx and Gilead Sciences resulted in zero cases of delayed neurotoxicity, including Parkinsonian symptoms and cranial nerve palsies, according to early results from a Phase 2 clinical trial reported yesterday.
While still preliminary, the data suggest the treatment could be a safer option than Carvytki, a currently approved CAR-T treatment from Johnson & Johnson and Legend Biotech. Parkinsonian symptoms and cranial nerve palsies have been reported in about 10% of patients treated with Carvykti, according to published clinical trial results.
Read more from STAT’s Adam Feuerstein.
Pharma portals seen to be opening up a dystopic era
The new online portals recently launched by pharma companies are ethically questionable and are ushering in a dystopic era of drugmakers essentially selling their treatments directly to patients, two researchers argue in an opinion piece.
Adriane Fugh-Berman and Judy Butler, researchers at PharmedOut, a Georgetown University research project focusing on inappropriate pharmaceutical marketing practices, note that sites such as Lilly Direct and PfizerForAll encourage patients to seek an instant consultation with a telehealth prescriber who will recommend a drug that can then be ordered through those sites.
“Oh, sure, there is some cooperative prescriber involved, from a purportedly independent provider of cooperative prescribers. But the thin camouflage of a white coat can’t hide the fact that working for a company tethered to a pharmaceutical manufacturer and prescribing the same drug to almost all comers isn’t practicing medicine,” they write.