Globus Medical gets FDA nod for ortho robot
Listen to the article 3 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: Globus Medical received 510(k) clearance

Listen to the article 3 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: Globus Medical received 510(k) clearance

Dive Brief: Less than a week after the Food and Drug Administration published best practices for transparency in machine learning-enabled medical devices, a leader with

Dive Brief: The Association for the Advancement of Medical Instrumentation updated its standard on ethylene oxide (EtO) sterilizers used in healthcare facilities, the organization said

Listen to the article 2 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: Medline has asked hospitals to

Dive Brief: Vyaire Medical, a company that makes ventilators and respiratory diagnostics equipment, has filed for Chapter 11 bankruptcy protection. Vyaire struggled to refinance its
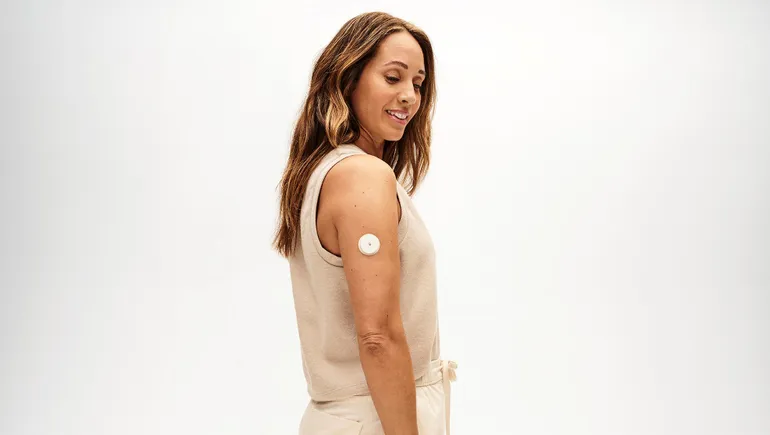
Dive Brief: Abbott said Monday it will launch two over-the-counter continuous glucose monitors after receiving clearance from the Food and Drug Administration. One product is

Dive Brief: Medtronic recalled a surgical navigation system used during neurosurgery procedures because of a software error that can cause physicians to use an incorrect

The Food and Drug Administration is grappling with a surge in the number of medical devices that contain artificial intelligence or machine learning features. The

Dive Brief: Abbott received clearance from the Food and Drug Administration for an over-the-counter glucose monitor. The device, called Lingo, first debuted in the U.K.

Dive Brief: Dive Insight: Akili was one of a few companies that sought to develop prescription apps, called digital therapeutics, that have received the FDA’s