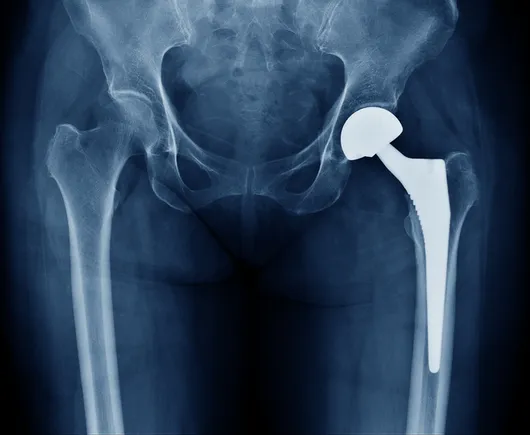
Zimmer pulls hip implant off the market due to fracture risk
Dive Brief: Zimmer Biomet will phase out sales of its CPT Hip System by December due to concerns about the risk of thigh bone fractures,

Dive Brief: Zimmer Biomet will phase out sales of its CPT Hip System by December due to concerns about the risk of thigh bone fractures,

Dive Brief: Senseonics and commercial partner Ascensia Diabetes Care received clearance from the Food and Drug Administration for the first implantable glucose sensor that can

Dive Brief: Smiths Medical recalled tracheostomy kits because of a manufacturing defect that could cause the balloon to separate from the inflation line. The recall,
Dive Brief: Bausch + Lomb is exploring a potential sale that would separate the eye care company from parent firm Bausch Health, the Financial Times

Roche plans to launch its first continuous glucose monitor (CGM) in Europe “in the coming weeks,” Sérgio Moreiras, the company’s international business leader for continuous

More than 40% of all artificial intelligence tools cleared or approved by the Food and Drug Administration lack clinical evidence, according to an article recently
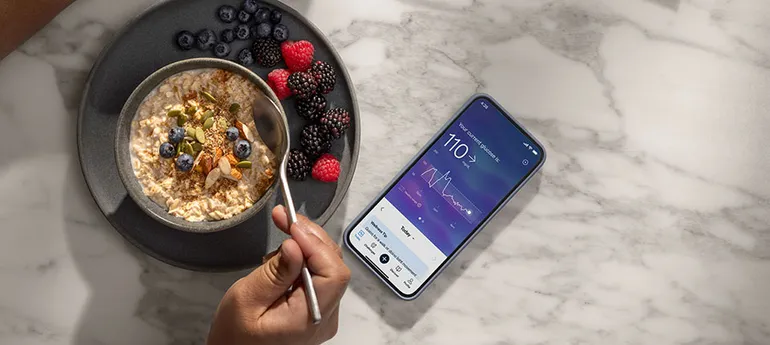
Dive Brief: Abbott rolled out its first over-the-counter glucose sensor in the U.S. on Thursday, just over one week after Dexcom began selling its competing

The Food and Drug Administration sent warning letters to four companies for selling devices that claim to clean continuous positive airway pressure (CPAP) machines but

Dive Brief: Embecta received 510(k) clearance from the Food and Drug Administration on Friday for its first insulin patch pump. The device can be used

Dive Brief: Penumbra will lay off 71 people working in its Immersive Healthcare business, according to a California Worker Adjustment and Retraining Notification notice obtained