
A ‘tie-breaker’ study of Abbott’s heart clip brings more questions than answers
A study meant to serve as a tie-breaker between two major trials of a heart clip made by Abbott may end up stimulating more debate

A study meant to serve as a tie-breaker between two major trials of a heart clip made by Abbott may end up stimulating more debate

A group representing molecular pathologists sued the Food and Drug Administration on Monday over its plan to regulate lab-developed tests. It’s the second legal challenge
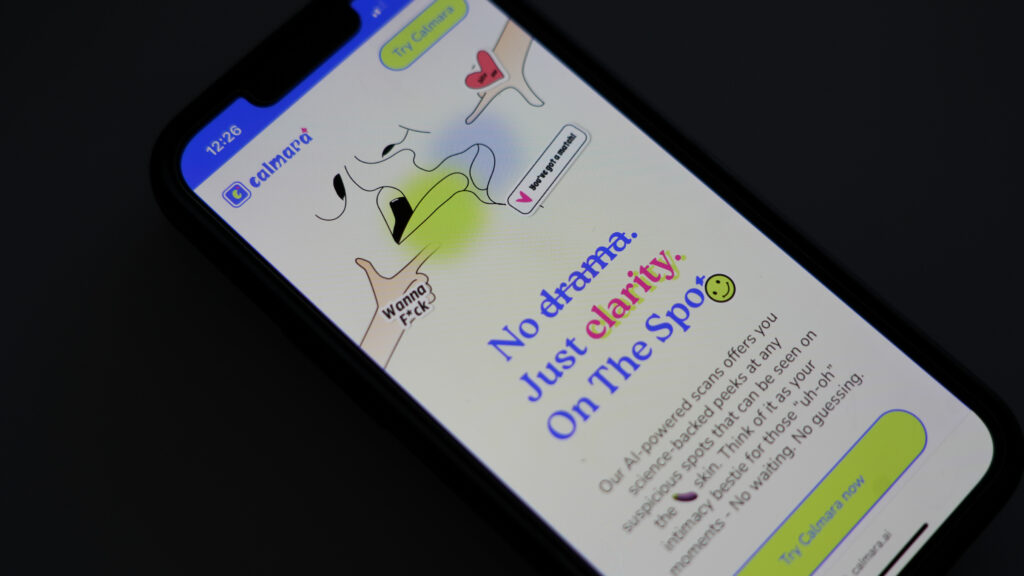
The company that claimed its AI model could identify sexually transmitted infections from a single penis picture was shut down by the Federal Trade Commission

The Centers for Medicare and Medicaid Services has finalized its rule easing reimbursement for medical device makers. Called Transitional Coverage for Emerging Technologies, the program

Retirements from the Food and Drug Administration are hardly shocking, especially in the wake of pandemic burnout. But when longtime medical device director Jeff Shuren
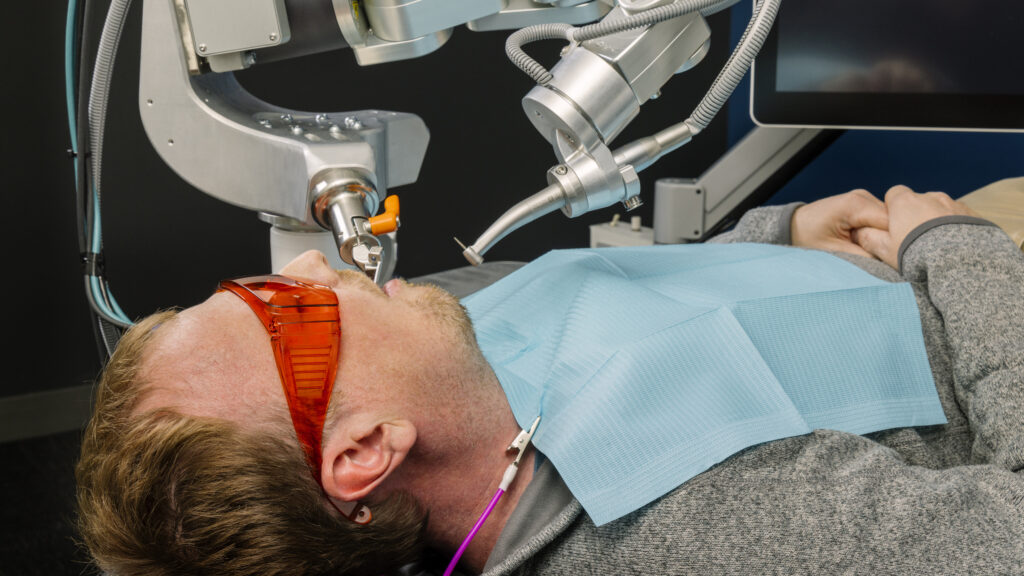
As the AI race in dentistry grows more heated, a startup called Perceptive is aiming to automate each stage of a dental visit, from imaging

Jeffrey Shuren, longtime chief regulator of medical devices at the Food and Drug Administration, announced to staff on Tuesday that he is leaving the agency,

When a new medical device hits the market, there’s typically still some uncertainty about whether it works. Device makers generally do not have to submit

Boston Scientific said on Tuesday it will buy Silk Road Medical, a medical device company treating stroke, for $1.16 billion. Silk Road Medical sells technology

The former CEO of Stimwave, a company that sold pain-relief devices with dummy pieces of plastic, was sentenced Monday by a New York judge to