
FDA posts early alert after Calyxo urinary stone device tied to death
Listen to the article 2 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: The Food and Drug Administration

Listen to the article 2 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: The Food and Drug Administration

Listen to the article 5 min This audio is auto-generated. Please let us know if you have feedback. Medtech companies used GTC 2025 this week

Dive Brief: Medtronic has recalled embolization devices collectively linked to reports of 17 injuries and four deaths, the Food and Drug Administration said Tuesday. The
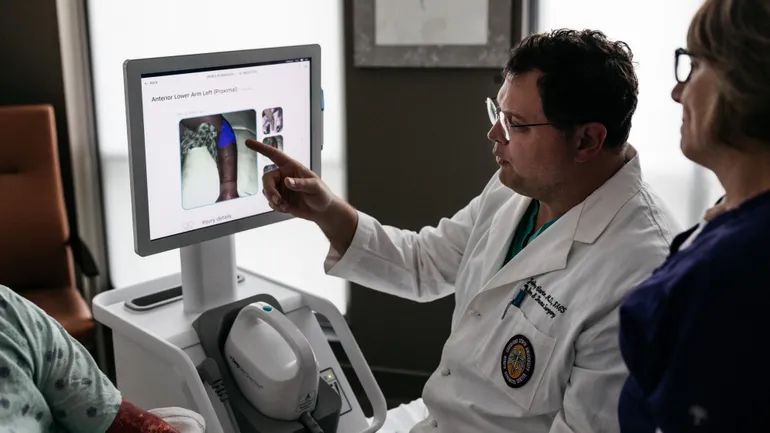
Dive Brief: Spectral AI’s Deepview System has outperformed burn physicians in identifying non-healing tissue, the company said Monday. Deepview achieved 86.6% sensitivity when identifying non-healing

Listen to the article 3 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: The Food and Drug Administration

Listen to the article 3 min This audio is auto-generated. Please let us know if you have feedback. Name: Sarah Romano New title: CFO, Vicarious

Dive Brief: The Food and Drug Administration has found Mid-Link Technology Testing copied the results of another study or created falsified or otherwise invalid data.
Listen to the article 4 min This audio is auto-generated. Please let us know if you have feedback. Dive Brief: ECRI has named insufficient governance

Dive Brief: Dexcom received a warning letter on March 4 after the Food and Drug Administration found problems at facilities in Arizona and California, the

Dive Brief: The Federal Trade Commission has challenged a private equity firm’s attempt to buy Surmodics for approximately $627 million, the regulator said Thursday. Surmodics