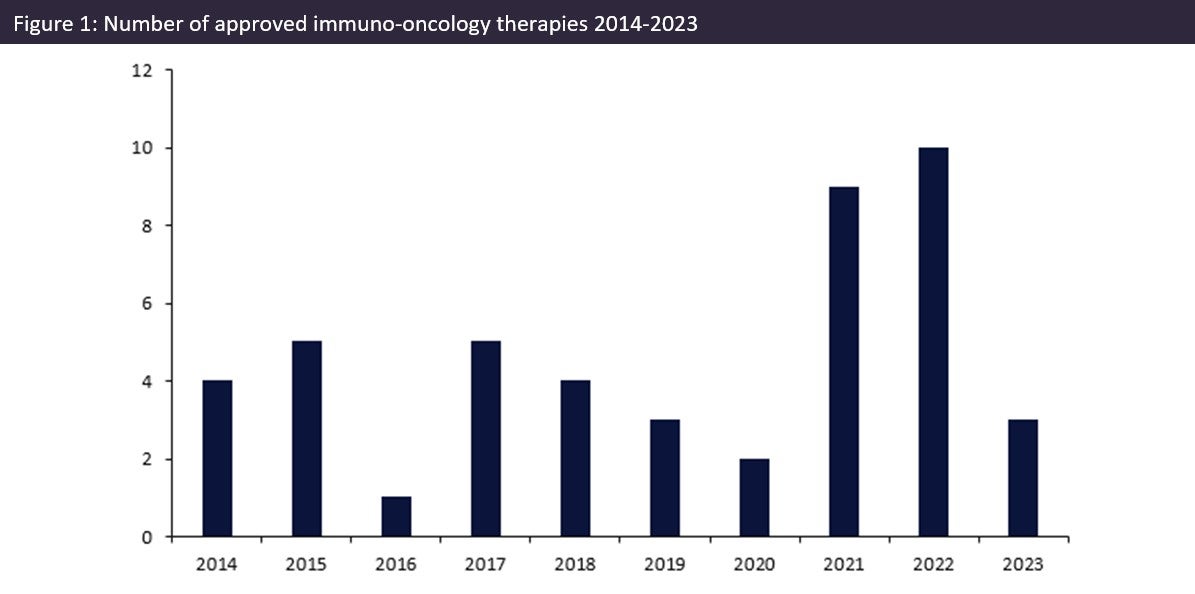
Over the last ten years, the number of approved innovator immuno-oncology therapies (immunotherapies) has increased, culminating in a record number of approvals in 2022 with ten new innovative drugs being approved. As oncology diagnoses increase across the globe, immuno-oncology therapies have come forward as a novel treatment category, outside of chemotherapy and radiotherapy, that can help combat aggressive forms of cancer.
Immuno-oncology is an umbrella term describing a variety of cancer treatments that include drugs such as cancer vaccines, immune checkpoint modulators, and adoptive cell therapies such as chimeric antigen receptor T-cell (CAR-T) immunotherapy and natural killer (NK) cell immunotherapy. All forms of immunotherapy function in a similar way, activating and utilising different components of the immune system to boost the body’s natural ability to recognise and fight cancer. The first officially recognised immuno-oncology therapy was Yervoy by Bristol-Myers Squibb, a monoclonal antibody that functioned as an immune checkpoint inhibitor and was approved in 2011 by the US Food and Drug Administration (FDA) for metastatic melanoma.
According to GlobalData’s Drugs database, the number of Immuno-oncology therapy approvals has been fluctuating significantly, rising, and falling over the last ten years. As seen in Figure 1 (above), an initial 25% increase in the number of approvals between 2014 and 2015 was followed by a decline of 80% in 2016, with a subsequent rise and another fall between 2017 and 2020 with a 60% decline over those four years. However, 2021 saw the number of approvals increase by over 300% and a further 11% increase in 2022, with ten immuno-oncology therapies being approved in a single year, a record number of approvals. This record-breaking cohort included therapies such as Carvykti by Johnson & Johnson, a gene-modified cell therapy that was approved for refractory multiple myeloma; another example is Hansizhuang by Shanghai Henlius Biotech, a monoclonal antibody approved for lung cancer and other solid tumours.
The large increase in the number of immuno-oncology therapies being approved over the last two years highlights their significance, especially since the Covid-19 pandemic led to delays in the review and approval of multiple drugs. If this peak in the number of approvals continues, it may mean that we see a shift in the types of oncology therapies making it to the market with immuno-oncology therapies making up a greater share. However, with only three therapies approved in the first half of 2023, it remains to be seen if 2022’s high number of approvals will be matched.
