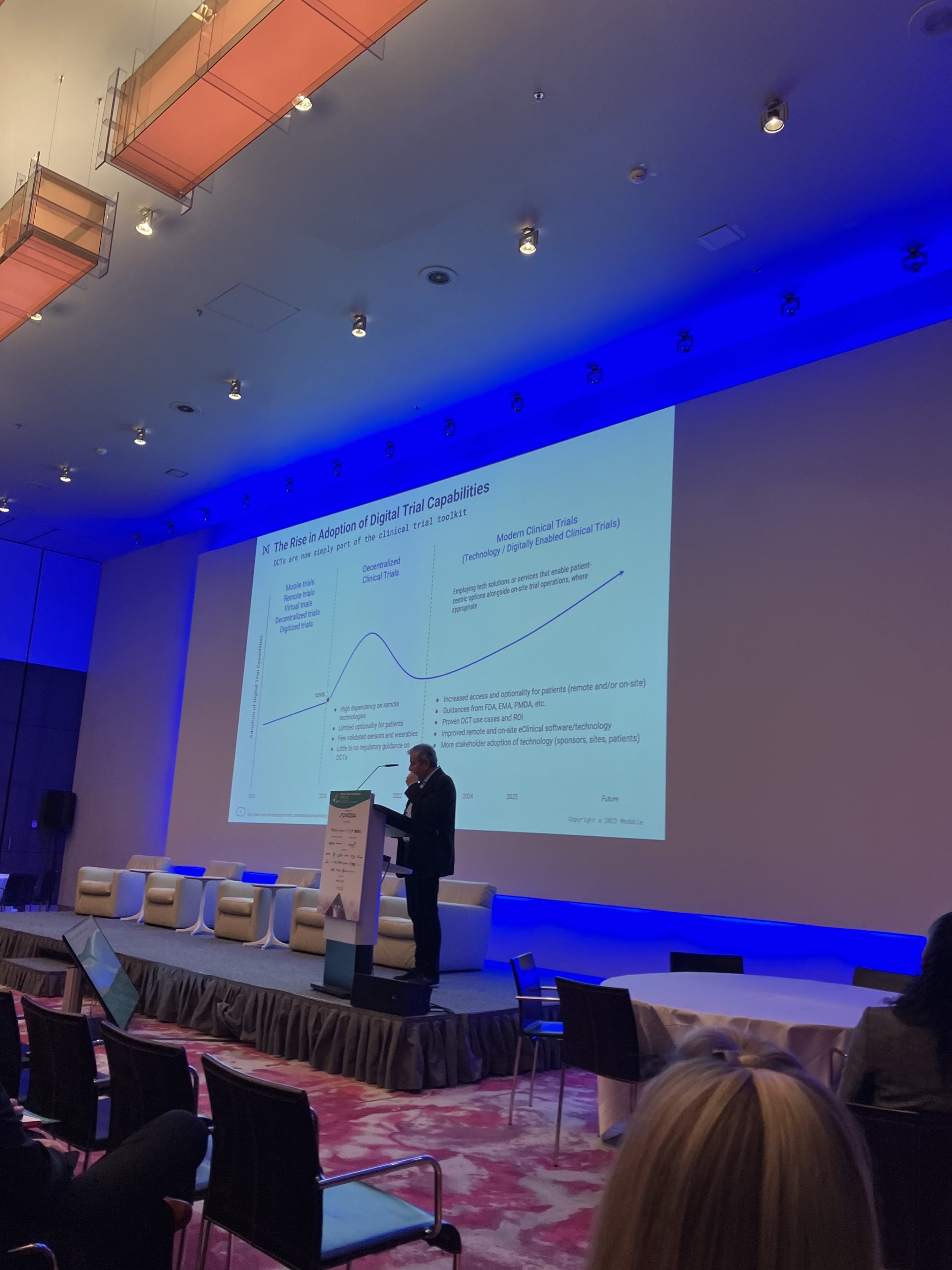
Building on the increased adoption of digital trial capabilities due to the Covid-19 pandemic, the future of clinical trials will see a further turn towards the use of technology solutions that will enable patient-centric options, says Stefano Ferrara, director of clinical science at BeiGene.
Ferrara was speaking at an opening session of the Clinical Trials in Oncology (CTO) Europe 2023 conference in Munich, Germany, taking place 28 – 29 November.
However, the future of digitally-enabled clinical trials will involve the combined use of tech solutions and services together with on-site study operations, which will continue to be used when necessary. This will improve access and optionality for patients enrolled in such trials, which will retain capacities for both remote and on-site operations, based on the presentation.
But unlike the Covid-19 pandemic, such operations will now include greater guidance from regulatory bodies such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Similarly, there will be an increased adoption of technology amongst stakeholder such as sponsors, sites, and patients.
The use of digital technologies in oncology-focused studies will be useful as they can tackle some of the field’s particular challenges. Since there is potential for severe side effects with investigational therapies in cancer, there is a need for rigorous safety monitoring over a longer period time, said Ferrara. Digital technologies can be used for remote patient visits that could also help cancer patients by reducing the emotional and physical burden involved in going to trial sites, added Ferrara. Moreover, the use of digital technologies will also expand the global reach of the trials.
Beyond this, new technologies such as artificial intelligence (AI) and liquid biopsies will also further the clinical development of new therapies. In clinical development, artificial intelligence (AI) could be used for enhanced data analyses and in designing trials while liquid biopsies can be utilised for an earlier detection and assessment of diseases.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
However, while many claim that AI will help with clinical development, it is important to remember that the community must be able to use such tools correctly, said Ferrara. He added that such tools need to first be understood well, comparing the situation to someone using a hammer without knowing how to use it properly. Similarly, tools such as liquid biopsies need to be validated and compared with tumour biopsies to make sure the technologies are used correctly, added Ferrara.
On a broader level, AI could also change the state of work in clinical development. According to Ferrara, AI could reduce the need for data verification, which could in turn push companies towards a more discussion-based model that would involve researchers and clinical sites.

Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
