What You Should Know:
– Cytovale, a medical diagnostics company revolutionizing early disease detection secures $100M in Series D funding round led by Sands Capital with participation from new investor Canada Pension Plan Investment Board (CPP Investments) and existing investors.
– The investment will fuel the expansion of Cytovale’s innovative sepsis diagnostic, IntelliSep, to more hospital emergency departments (EDs) and health systems nationwide.
IntelliSep: Transforming Sepsis Detection in the Emergency Department
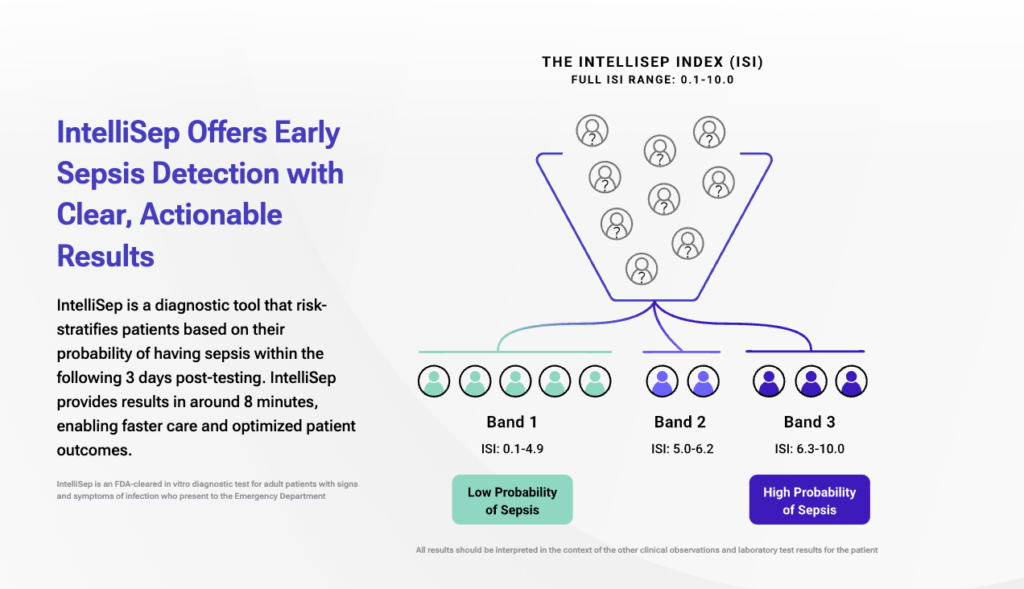
Sepsis, a life-threatening condition caused by the body’s overwhelming response to infection, often presents in the emergency department (ED). IntelliSep is the first and only FDA-cleared cellular host diagnostic specifically designed for use in the ED, where over 80% of sepsis cases initially appear.
This innovative test provides clinicians with a unique window into the biological mechanisms driving sepsis, enabling rapid and confident diagnosis. With results available in approximately eight minutes, IntelliSep empowers care teams to deliver timely and targeted treatment, potentially saving lives.
Early Success and Improved Outcomes
Since its launch in August 2023, IntelliSep has demonstrated significant clinical impact. Our Lady of the Lake Regional Medical Center in Baton Rouge, Louisiana, an early adopter of the technology, has reported impressive results:
- Faster Treatment: Earlier detection of sepsis through IntelliSep has led to treatment initiation more than 60 minutes faster.
- Reduced Mortality: The hospital observed a 30% decrease in the risk-adjusted mortality index for sepsis patients.
- Shorter Hospital Stays: Patients tested with IntelliSep spent 1.28 fewer days in the hospital.
- Cost Savings: The hospital realized a savings of $1,400 per patient tested with IntelliSep.
Expanding Access to Life-Saving Technology
This new funding will enable Cytovale to:
- Accelerate Commercial Expansion: Bring IntelliSep to more hospital EDs and health systems nationwide, increasing access to this life-saving diagnostic tool.
- Build on Clinical Success: Further validate the clinical and economic benefits of IntelliSep through continued research and data collection.
- Advance Early Detection Technologies: Invest in the development of new diagnostic solutions for fast-moving and immune-mediated diseases.
“Sepsis has historically been one of the most challenging and costly conditions for hospitals to manage due to the lack of rapid, objective diagnostic tools. Thankfully, that’s finally changing with IntelliSep, which holds the potential to transform sepsis care in the same way troponin tests did for cardiac care and rapid CT scans did for stroke diagnosis,” said Cytovale CEO Ajay Shah, PhD. “Cytovale is growing at an astounding pace to meet demand from other health systems looking to tackle this deadly condition. The additional investment will enable us to quickly scale across the U.S. with greater agility to serve health systems and their patients.”