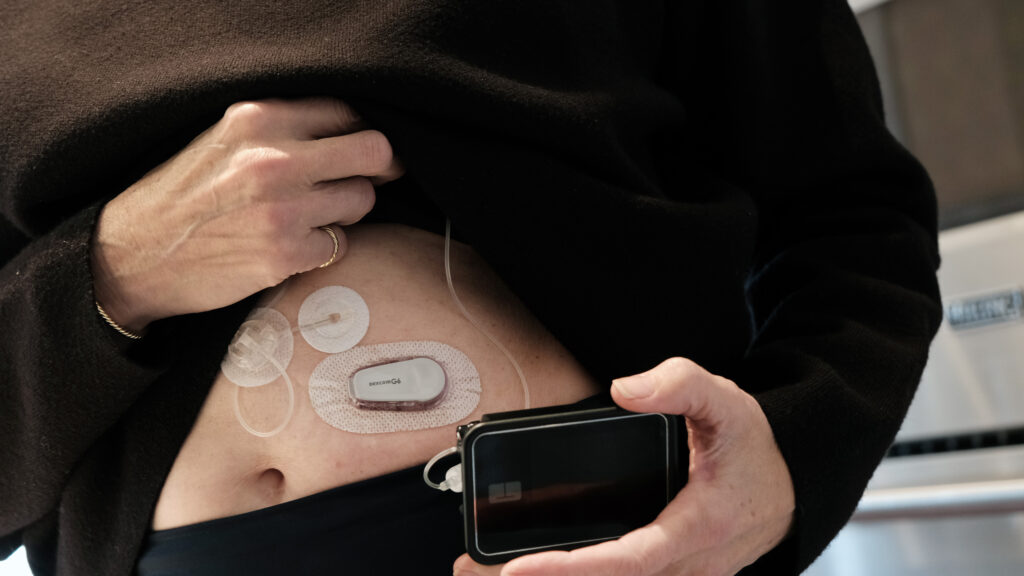
When Dana Lewis built her first artificial pancreas in 2014, she — and millions of other people with type 1 diabetes — didn’t have another option. Back then, diabetes device manufacturers hadn’t closed the loop between the blood glucose sensors that help determine a person’s next insulin dose and the pumps that deliver it. So she took the reins, hacking her existing devices to build a DIY system that could automate her insulin doses.
Since then, the community of DIYers with type 1 diabetes has grown to the thousands. But today, they have alternatives: Device manufacturers have caught up with their own, FDA-cleared closed-loop systems, and automated insulin delivery is becoming more common. In a world with these commercial options, does the DIY artificial pancreas still have a place?
advertisement
The answer, said pediatric endocrinologist Greg Forlenza in a debate with Lewis on Friday at this year’s American Diabetes Association scientific sessions, is no. With four approved systems on the market, he argued, there’s no need to take on the risk of linking together a hodgepodge of tools with an algorithm that itself is unapproved by the FDA.
“There’s no point to it,” Forlenza, who has consulted for and conducted research supported by major diabetes device manufacturers at the Barbara Davis Center, told STAT before the session. “It’s not offering anything beyond what is offered directly through traditional commercial means.”
Lewis, who ultimately shared her DIY artificial pancreas code for others to use, begs to differ. She still uses her own system because the FDA-cleared commercial systems aren’t cutting it for her.
advertisement
It’s true, she acknowledged, that glucose outcomes for people using commercial and DIY systems are similar. That includes clinical measures like A1C, which reflects average blood glucose, and time in range, the percentage of hours spent with real-time blood glucose values within healthy limits.
But for people with type 1 diabetes, who must wear and manage their insulin systems 24/7 to stay alive, that only tells part of the story.
“There’s always this balance between what are the glucose outcomes, and what is the amount of work to put in,” Lewis told STAT before the debate. For her and many DIYers, the ability to custom-tune their insulin delivery algorithms — to reduce the number of times they have to touch their device, or respond to an alarm, or enter a meal to help the system determine its next insulin dose — can offer significant quality of life improvements. In clinical research, “that’s something that we don’t quantify right now,” she said.
On the advanced end, that flexibility has allowed some intrepid DIYers to test fully-automated systems. Existing artificial pancreases still require the user to input carbohydrate counts and meals to help the algorithm determine the right insulin dose. But Lewis pointed to a pilot study of 10 DIY users that showed good outcomes without that extra effort. DIY, she argued, is still an important force for accelerating diabetes research and technology.
“There’s things that we’ve been learning since 2014 that need to feed into how we build, use, and teach people to use these systems,” said Lewis.
But it’s precisely that kind of boundary-pushing that concerns Forlenza. He points out that researchers and manufacturers are also working toward fully closed-loop systems, “but we have to study it appropriately,” he said. “Because it stands the chance of hurting someone, and we have to make sure that it doesn’t.”
The systems on the market today have the advantage of having been tested in clinical trials before being released to the general public, are warrantied, and have the support of industry teams if something goes wrong. And over time, they’ve presented some of the options that DIYers have clamored for, like an insulin pump that delivers medication through a patch instead of a tube.
Without typical safeguards, Forlenza said, DIY is too complex for most patients and physicians to rely upon. Two thirds of patients with type 1 diabetes, he explained, have their care managed by primary care physicians, not endocrinologists, and many are still not using insulin pumps. To expand automation and its benefits to as many patients as possible, he said, “they need something that is as simple and off the shelf as prescribing a drug.”
There could be a middle ground, though. DIY may be an essential option for thousands of people with type 1 diabetes, but it would be difficult to make it a solution for millions. “DIY is pretty niche. I love it. I think it’s great,” said Aaron Kowalski, CEO of the Juvenile Diabetes Research Foundation and past DIYer. “But I think the real true end of this story is interoperability versus fully sealed-up systems.”
If manufacturers made pumps, sensors, and algorithms that were built to work interchangeably, people with type 1 diabetes could mix and match the best FDA-cleared options for them — “the benefits of DIY, but for a mass market,” Kowalski said. The FDA has made a concerted effort, starting in 2018, to support a new class of diabetes tools that could ultimately play together.
“The notable feature of the do it yourself community is that they are willing to try things out and take risk in trying things out that is atypical for a regulated medical device,” Howard Look, the CEO of Tidepool, told STAT in 2022 about its interoperable insulin dosing algorithm. The nonprofit received clearance for Tidepool Loop, which is based on an open source DIY algorithm, in January.
“We’re trying to strike that balance,” said Look. “Which is how do you keep that same rate of innovation, that same rate of improvement that the DIY community knows and loves, but also make it clear not just to the agency but to the public, here’s how we know that this thing is safe.”
But in practice, these new device classes haven’t manifested into more interoperable, plug-and-play options. When Tidepool initially signaled its intent to bring Loop through the FDA, both Medtronic and Insulet agreed to make their insulin pumps compatible — but they later pulled out, leaving the algorithm in limbo.
“There are no technical barriers. There are no regulatory barriers,” said Joseph Cafazzo, a digital health researcher at the University Health Network in Toronto. It’s business interests that are getting in the way of interoperability. “It’s just not in the interest of some of the more prominent market leaders to cede the potential of bleeding off their customer base to third parties. They want the full stack ability to continue to dominate the market.”
“It’s going to take device makers stepping up and stepping forward to actually participate with this concept of choose your own device from each of the three categories for it to actually happen in the real world,” said endocrinologist Aaron Neinstein, vice president of digital health for UCSF Health and co-founder of Tidepool.
It’s not just the devices that manufacturers want to control: It’s the data they produce. The glucose and insulin dosing data produced by CGMs and pumps, along with information about individual users and their lives, are the raw material necessary to design best-in-class insulin dosing algorithms.
When the walls between devices come down, it opens up that data for more innovation — like in the data commons that Lewis created for DIY users. The ultimate goal, she said, is to build better tools for people with diabetes.
“Most of us in the DIY and open source community are doing it for ourselves, but then also saying, ‘Please make things better for everybody else.’ It could be through DIY or could be through better algorithms, better safety, better interoperability, whatever. We just need to do better for everybody.”