By Vivienne Raper, PhD
Emerging biologics such as multispecific antibodies, mRNA vaccines, and viral vector–based gene therapies are more complex than established biologics such as monoclonal antibodies and recombinant proteins. Consequently, emerging biologics pose unique product stability challenges. Meeting these challenges—and preventing adverse consequences such as dangerous immune reactions in patients—requires a deeper understanding of product stability. More to the point, it requires improved product stability technologies.
Today, various product stability technologies are being developed. To gather more information about them, GEN has consulted with several experts who are collecting data earlier in development, using new instrumentation to monitor the stability of adeno-associated virus (AAV) capsids, and creating new formulations that remain stable even with high concentrations of drugs.
Assessing stability early
“Products belonging to new therapeutic areas are of great interest, but there’s not a lot of knowledge about them,” says Roberta Bucci, fellow scientist, bioanalytical development, Catalent Biologics. She adds that manufacturers that lack sufficient knowledge may be unable to resolve stability testing problems within the limits imposed by project timelines.
“If you have an issue with your late-phase product, it’s too late to take a step back to fix or improve the product,” she emphasizes. To help manufacturers avoid this problem, she recommends running preliminary stability studies as early as possible in development: “In my view, you need to set up all the studies needed to get a good understanding of the product before moving onto any subsequent phase.”
These early studies might include using small-scale batches to predict product stability under specific storage conditions. Introducing a structured training program into the laboratory is also important, she says, to ensure personnel use the correct techniques to manipulate the product. She adds that Catalent, as a contract development and manufacturing organization, works with new customers to assess whether new products need additional work before they can advance further into development.
Adopting new technologies
A key trend in the industry is the identification of stability parameters that need to be assessed during development. Such parameters, Bucci says, include the identity, size, size distribution, and concentration of subvisible particles. According to Bucci, these parameters are of “great interest” because they can predict product aggregation.
This clumping of drug product has the potential to generate immune reactions in patients, adds Bernardo Cordovez, PhD, CSO and co-founder of Halo Labs.
“Whether a biologic is an AAV-based gene therapy or a monoclonal antibody, it can aggregate quite a bit,” he notes. “If it does, it can trigger your immune system and come under attack. Even if the biologic had been working well otherwise, it will end up being cleared. It will stop working.” In the worse cases, he continues, the physical instability of the drug can cause severe allergic reactions.
A solution to this problem, Bucci suggests, is early assessment of product stability, ideally using multiple nondestructive techniques to avoid destroying small, valuable samples. Bucci mentions that two Beckman Coulter instruments (the HIAC 9703+ Pharmaceutical Particle Counter and the FlowCam 800 Flow Imaging Microscope and Particle Analyzer) are useful for studying subvisible particles in small sample volumes.
Using microscopy and membranes
Other companies are also developing new instruments for assessing aggregation. One of these companies is Halo Labs, which offers Aura GT, an instrument developed for subvisible particle detection in gene therapies. Aura GT uses an automated microscope and a membrane plate to count, size, and identify subvisible particles.
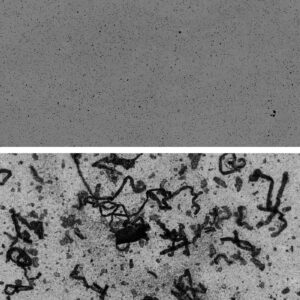
Cordovez explains that the instrument works by imaging a membrane with well-defined pores and then using the membrane to filter a sample (for example, a sample of an AAV-based product). Large particles (bigger than 2 μm) are left behind on the membrane. These particles can be imaged using fluorescence and an additional light source to establish particle counts and sizes, and to determine whether the particles are viral capsids, external contaminants, or even excipient aggregates.
“Viral vectors are much more biologically complex than their predecessors,” Cordovez notes. “Not only does each viral particle have a protein shell, but it also has an important and sticky genomic component inside. … So, if you have particles, you want to know [whether they are] viral particles that have been leaking and aggregating, or whether [they have been formed by] excipients that were meant to keep the viral particles stable.”
A major selling point for Aura GT, Cordovez suggests, is that filtering product nondestructively through a membrane means it can analyze particles in sample volumes as small as 5 μL. “Aura GT was customer-inspired,” he relates. “People were interested in asking more questions and in using samples that were smaller in volume. The low volume is incredibly important for viral vectors because yields are three to five orders of magnitude lower than for established biologics, and because AAVs have a more complex nature.”
Studying AAV breakdown
Unchained Labs is another company that has entered the growing market for gene therapy stability testing. Back in 2020, Unchained Labs launched an AAV stability application for the company’s Uncle screening platform.

According to Kevin Lance, PhD, director of viral vector marketing at Unchained Labs, the Uncle platform allows rapid stability assessment using three different methods: full-spectrum fluorescence, static light scattering, and dynamic light scattering. He notes that the platform can assess long-term storage stability, which is becoming increasingly important to the industry, by rapidly increasing the temperature of a product sample. If, for example, it is unclear whether a product could last at 4°C for six months, the platform could raise the temperature of a product sample from 15° to 95°C. Stability information can be generated within two hours.
“The broader trend in the industry is for longer storage at more desirable temperatures,” Lance observes. The first commercially successful AAV vectors, he recalls, imposed several stringent “don’ts.” Don’t keep the vectors for more than two weeks. Don’t shake them. Don’t store them in the freezer. Lance suggests that for clinicians, these restrictions only added to the stress of administering a million-dollar treatment. Fortunately, there were fewer “don’ts” by 2017, the year Spark Therapeutics’ gene therapy Luxturna was approved in the United States. As Lance notes, Luxturna is shelf-stable and storable in the freezer.
The Uncle AAV application, Lance explains, allows the process of AAV breakdown to be monitored. This process, which includes the unfolding and breaking down of the AAV protein, results in the capsid “exploding like the Death Star.” The unfolded proteins are sticky, and they tend to aggregate.
Another instability pathway that can be monitored, Lance says, is one that allows DNA to escape the intact capsid before breakdown and aggregation occur. He asserts that when this pathway is monitored with the Uncle platform, it is possible to evaluate stability behavior before aggregation happens, and even before capsids fall apart.
Managing high concentrations
High-concentration biologics are “absolutely becoming more common,” says Deborah Bitterfield, PhD, founder and CEO of Lindy Biosciences. She adds that these biologics present formulation and stability testing challenges. This thought is seconded by Bucci, who states, “When you’re testing highly concentrated products, you might hesitate to dilute your samples. You wouldn’t want your dilutions to distort your findings.”
Instead of chromatography, which may require sample dilution, Bucci recommends using a system like the CTech SoloVPE system from Repligen. With this system, there is no need to dilute before testing. Using a technique that doesn’t require dilution ensures that
behaviors such as aggregation aren’t disturbed by the preparation process, and that a more accurate view of product attributes can be obtained.
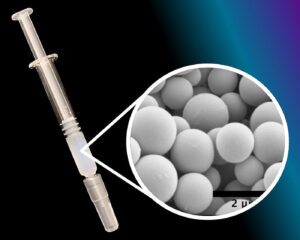
Another challenge is keeping high-concentration formulations stable for subcutaneous injection. “There’s a huge trend in the industry toward subcutaneous delivery for monoclonal antibodies,” Bitterfield says. “It allows patients to deliver medication at home.” But if monoclonal antibodies or bispecific antibodies need to be given in higher doses to be effective, the subcutaneous delivery of these therapeutics can be difficult.
Lindy Biosciences is a spinoff company from Duke University, where researchers have developed Microglassification, a dehydration technology. According to Bitterfield, it creates dense, spherical particles of solid protein. The Microglassified dry powder has a two-year shelf life under refrigerated conditions, and it can be suspended in an oily non-water substance that allows it to be injected using a standard prefilled autoinjector syringe. Once the dry powder is injected subcutaneously, it dissolves.
Lindy Biosciences is at a preclinical stage of development with undisclosed partners, and it expects to be at the clinic with its technology by 2025.
