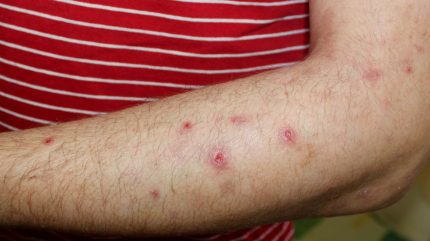
The US Food and Drug Administration (FDA) has approved Galderma’s Nemluvio (nemolizumab) as a pre-filled pen for subcutaneous injection to treat adults with prurigo nodularis, a chronic skin disorder indicated by multiple, firm, pink-coloured nodules on the extremities.
Nemluvio received FDA breakthrough therapy designation in December 2019 and priority review in February 2024.
The FDA is grounded in the positive outcomes from the Phase III OLYMPIA studies.
These trials showed that Nemluvio significantly improved itch and skin nodules by week 16, with some patients experiencing itch reduction as early as week 4.
The Phase III OLYMPIA 1 and OLYMPIA 2 trials assessed Nemluvio’s efficacy and safety in more than 500 subjects.
Administered subcutaneously every four weeks, the therapy met both primary and key secondary endpoints.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
It also confirmed a rapid reduction of itch and sleep disturbance within the first four weeks of treatment.
Nemluvio was generally well tolerated with its safety profile in line with those seen in the Phase II trial and across both OLYMPIA trials.
The US regulator is also reviewing a biologics licence application for Nemluvio for moderate-to-severe atopic dermatitis, with a decision expected later in 2024.
Nemluvio specifically targets IL-31 cytokine signalling, which is known to cause itch and is involved in inflammation and skin tissue hardening in prurigo nodularis.
It was originally developed by Chugai Pharmaceutical. Galderma obtained exclusive development and marketing rights for nemolizumab globally, excluding Japan and Taiwan.
Galderma CEO Flemming Ørnskov stated: “The US FDA’s rapid approval of Nemluvio in prurigo nodularis is a first step in achieving its blockbuster platform potential and reinforces our leadership in therapeutic dermatology.
“We’re confident in the impact this first-in-class therapy will have for patients with prurigo nodularis who urgently need more treatment options and look forward to potentially bringing Nemluvio to patients with other itch-related skin diseases in the near future.”

Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
