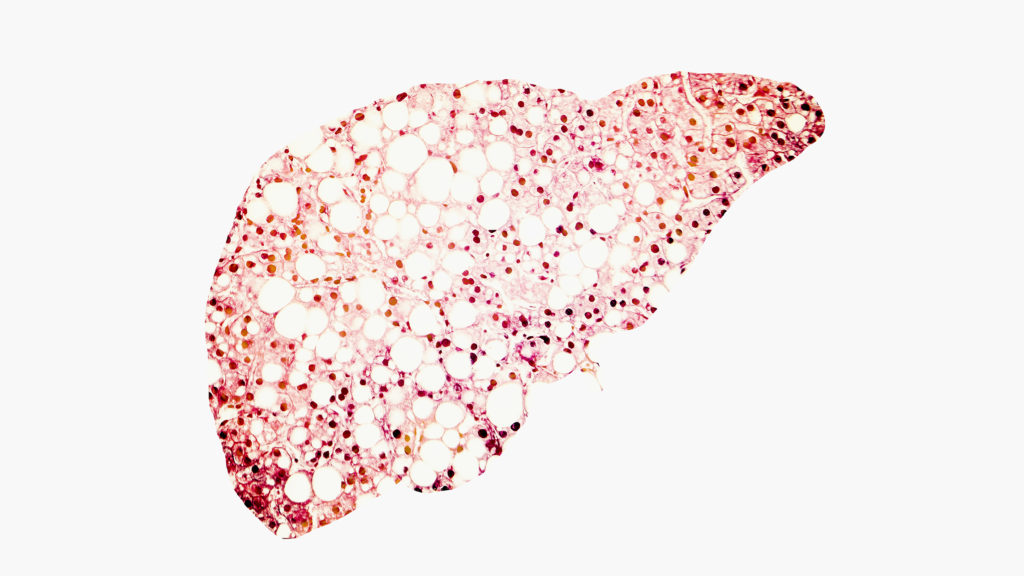
The Food and Drug Administration rejected Intercept Pharmaceuticals’ investigational treatment for NASH on Thursday, derailing what would have been the first approved medicine for a prevalent liver disease.
The FDA’s rejection made clear that reapplying for approval would require waiting about three years for a large, ongoing clinical trial to conclude, the company said in a statement. Instead, Intercept plans to cease its investments in NASH research, abandoning a field of study whose promise once made it a multibillion-dollar company.
advertisement
“While this is clearly not the outcome that we have worked toward, I’m proud of the impact that Intercept has made to move the science of NASH forward and bring the field closer to a treatment option,” CEO Jerry Durso said in a statement.
Unlock this article by subscribing to STAT+ and enjoy your first 30 days free!