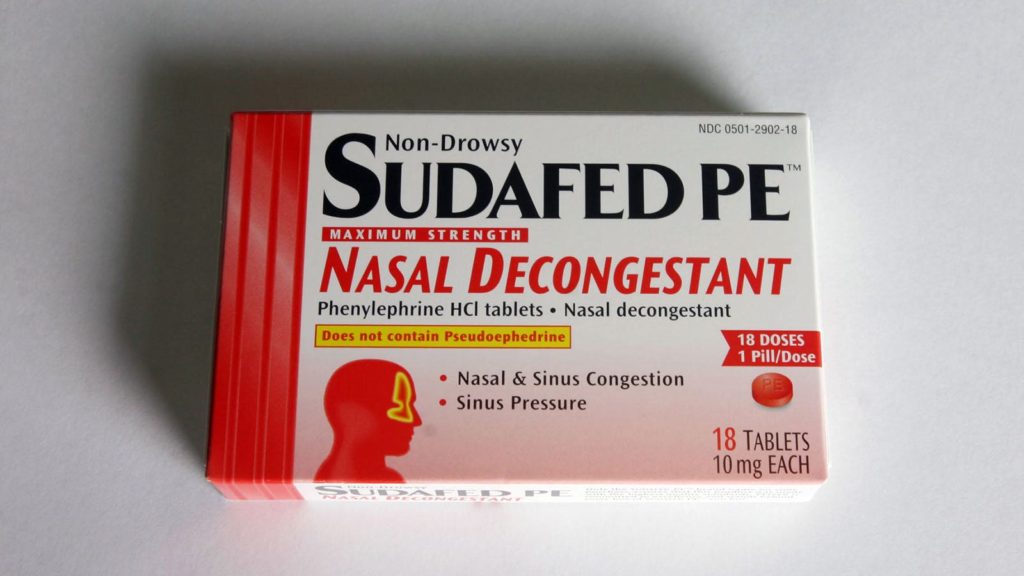
When pseudoephedrine moved “behind-the-counter” nearly 20 years ago, it left oral phenylephrine (with brands including Sudafed PE and Suphedrine PE) as the only nasal decongestant available without pharmacy assistance. But there’s one big problem: phenylephrine doesn’t work, the FDA has finally determined.
FDA reviewers released the results of their long-running review of the evidence this week as background for a meeting of the Nonprescription Drugs Advisory Committee to be held on September 11 and 12.
The drug’s over-the-counter (OTC) indication rests on meeting criteria for safety — which hasn’t been questioned and won’t be discussed by the FDA advisory committee — and “a reasonable expectation that, in a significant proportion of the target population, the pharmacological effect of the drug, when used under adequate directions for use and warnings against unsafe use, will provide clinically significant relief of the type claimed.”
When weighed by that standard, “we have now come to the initial conclusion that orally administered PE [phenylephrine] is not effective as a nasal decongestant at the monographed dosage (10 mg of PE hydrochloride every 4 hours) as well as at doses up to 40 mg (dosed every 4 hours),” the FDA documents noted.
The agency is weighing whether to scuttle the indications for phenylephrine hydrochloride and phenylephrine bitartrate due to lack of efficacy.
“However, we are concerned about avoiding potential unintended consequences,” the reviewers added. “We anticipate that any action taken by the Agency in this regard will have significant downstream effects, including effects on both industry and consumers, because the only other oral decongestant listed in the CCABA [Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic Drug Products for OTC Human Use] Monograph is pseudoephedrine, which is now regulated as a ‘behind-the-counter’ product under the Combat Methamphetamine Epidemic Act of 2005.”
In response to that law, most OTC decongestants switched from pseudoephedrine to phenylephrine.
If phenylephrine is off the market, it would keep people from unnecessary costs and delay in care from taking a drug that has no benefit and avoid the risks of potential allergic reactions or other side effects, especially from taking more in order to seek some benefit.
But, people will need to learn to “make the appropriate choices for alternative treatments,” the FDA document noted, albeit without spelling out what that should be.
FDA started looking at the efficacy of oral phenylephrine in response to a 2007 citizen’s petition and held an advisory committee meeting on the issue that same year.
In addition to the meta-analyses of the original studies supporting approval, that 2007 meeting included industry presentations of “new bioavailability data that show that <1% of an oral PE dose is systemically available in an active form as well as clinical information from two environmental exposure unit studies that suggest that PE is not more effective than placebo.”
Still, the advisory committee had called for more clinical data before a final decision would be made regarding the effectiveness for patients ages 12 and older.
A second citizen’s petition from the same group in 2015 requested that, based on large clinical trials conducted in the interim, phenylephrine hydrochloride and phenylephrine bitartrate be reclassified as not generally safe and effective, which would require their exit from the market.
Because the adolescent and pediatric monograph was based on historical data and extrapolation of adult data, the FDA reviewers noted that “any recommendations made regarding the efficacy of oral PE in adults would apply to its use in adolescents and children.”
Likely to figure prominently in the discussion are four studies:
- Two allergen challenge chamber studies from 2009, both finding that 10- or 12-mg phenylephrine didn’t reduce nasal congestion score compared with placebo
- A 2015 study of adults with seasonal allergic rhinitis, in which none of the groups getting fixed phenylephrine hydrochloride doses of 10, 20, 30, or 40 mg had a statistically significant change from baseline in instantaneous or reflective nasal congestion scores compared with people getting placebo
- A 2016 trial in adults with seasonal allergic rhinitis, in which 30-mg modified-release tablets of phenylephrine hydrochloride didn’t improve mean change from baseline during the entire treatment period in daily reflective nasal congestion score compared with placebo
An industry representative group, the Consumer Healthcare Products Association, argued for keeping the drug, citing the “totality of the scientific evidence” with pre-existing studies to support efficacy. They criticized the more recent clinical studies as having “important limitations (e.g., inadequate blinding and concomitant use of an antihistamine) and were conducted using a study population that is not appropriate to evaluate the efficacy of PE for OTC use.”
Their briefing document posted ahead of the advisory committee meeting cited the importance of “direct, on-shelf access to phenylephrine as a safe, effective oral nasal decongestant since symptom severity, product availability (both the number of product options and the availability of stores that offer them), and consumer preference may vary.”
The stakes are high, as one nationally representative consumer study of 100,000 U.S. households showed about half purchased OTC medications containing phenylephrine over the course of the year, and most of those did so multiple times a year.
Please enable JavaScript to view the