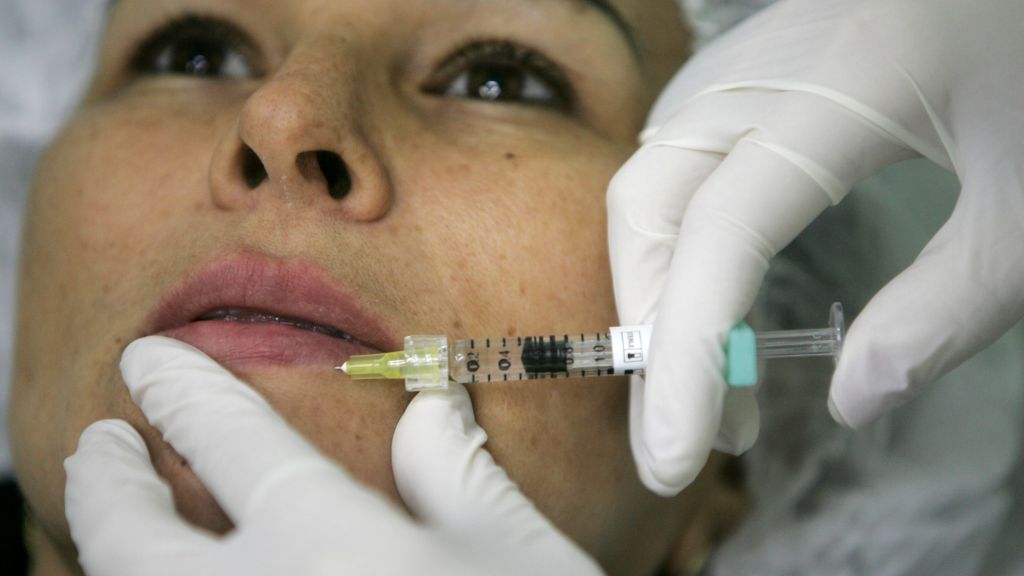
The Food and Drug Administration has scolded Merz Pharmaceutical for making misleading claims on Instagram about the safety and effectiveness of an injectable treatment for smoothing facial lines, the fifth time this year the agency has taken a pharmaceutical company to task for its marketing.
The regulator criticized Merz for a few reasons concerning Xeomin, which is promoted as an alternative to Botox for combating wrinkle and frown lines. The agency, for example, cited an Instagram post on an account belonging to Nate Berkus, a prominent interior designer, which highlighted various benefits but made it very difficult for consumers to read information about any risks.
advertisement
The agency noted that the reel begins with an “attention grabbing” shot of Berkus dancing to music and getting “ready for a big night out,” but the required risk information only appears at the very end — three seconds after the screen had gone black — in “small and difficult to read” lettering. The FDA argued this is unlikely to get the attention of a viewer.
STAT+ Exclusive Story
Already have an account? Log in


This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts.
Already have an account? Log in
To read the rest of this story subscribe to STAT+.