Dive Brief:
- The Food and Drug Administration is seeking examples of artificial intelligence and machine learning models that can identify and predict freezing of gait events related to Parkinson’s disease.
- Freezing of gait is a temporary loss of forward movement while walking. These episodes affect people’s quality of life and daily activities, but they can be difficult to measure because they often happen when patients are outside of a clinic or hospital setting.
- By testing these models against its own data, the FDA hopes to better understand the ability of these technologies to provide digitally derived endpoints that could help with early disease detection and prevention or support treatment and care in the home.
Dive Insight:
As digital health technologies proliferate, the FDA is taking a closer look at how they can capture information about a person’s health outside of a clinical setting. For Parkinson’s disease, a handful of wearable technologies have already been cleared to track symptoms.
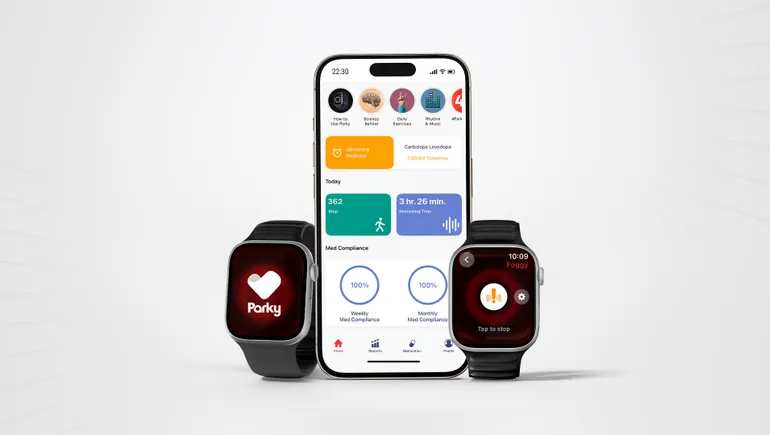
Apple published study results in 2021 evaluating its Motor fluctuations Monitor for Parkinson’s disease, which uses sensors to track fluctuations in resting tremor and dyskinesia. Later that year, a company called H2O Therapeutics received 510(k) clearance for its Parky app that sends Apple Watch data to a patient’s doctor. Last month, H2O received clearance for another app that provides haptic feedback when people experience freezing of gait symptoms.
Other cleared apps that use Apple Watch data include Rune Labs’ StrivePD software and an app developed by digital health company NeuroRPM.
Nearly 1 million people in the U.S. are estimated to have Parkinson’s disease, according to the FDA.
“With the fast-paced innovation and proliferation of DHT capabilities, including the use of artificial intelligence, the healthcare and technology ecosystems have a responsibility to ensure and support the safety, effectiveness, and privacy of digitally-derived endpoints,” the agency said in a summary of the challenge.
To start, the agency will ask developers, innovators and researchers to submit AI models before August 2. They can use public data sources or bring their own data.
Then, the FDA will pick the top performers from that group, and test those models against its own freezing of gait data. Its goal is to determine best practices for using AI models to develop and validate measures from wearable devices and smartphones.
The challenge is being hosted by the FDA’s Digital Health Center of Excellence, Office of Science and Engineering Laboratories and Office of Digital Transformation’s precisionFDA.