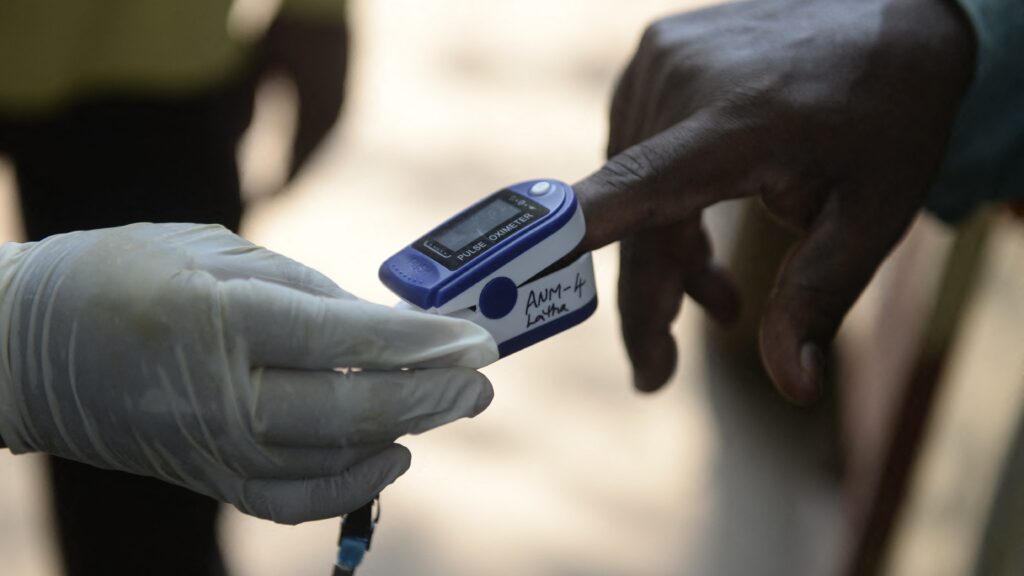
Work by device manufacturers to improve the performance of pulse oximeters on people with darker skin has progressed little since the Food and Drug Administration asked manufacturers in 2013 to voluntarily test the devices on more diverse skin tones, according to a study published Monday in JAMA. The study and a related editorial suggest clearer guidance, enforcement, and possibly legal action may be necessary to ensure the devices work well on all skin tones.
The new research analyzed paperwork submitted for FDA approval to market a device for 767 oximeters that had been approved between 1978-2024 and had accessible information about performance testing. The authors found references to skin tone, pigmentation, or race in 4% of the documents submitted before the FDA made its suggestion to diversify skin tone testing in 2013. That number rose to only 25% of submissions after the guidance was released — a number the authors said was still far too low and shows how little has been done to fix a problem — poorer performance on darker skinned patients — known for decades.
advertisement
“It is just utterly baffling to me that we had those reports in 1987, in 1990, in 2005, in 2007, in 2020, and we as a health care system have failed to fix it,” said Theodore J. Iwashyna, one of the study authors and a practicing critical care physician and professor at Johns Hopkins University. “Our patients need better devices now.”
Even though there was an increase in use of terms involving skin tone, race, and ethnicity, the researchers found repeated inconsistencies and much confusion in how the terms were defined and used.
“Sometimes these were combined in interesting ways,” said Kadija Ferryman, assistant professor in the Department of Health Policy and Management at the Johns Hopkins Bloomberg School of Public Health and the paper’s lead author. “One of the summaries we examined mentioned including people in performance testing with ‘light skin tones of China origins.’ There is a lot to unpack there—what does ‘China origins’ mean? How is it defined?”
advertisement
Ferryman called the lack of better handling of the issue by manufacturers seeking clearance for their devices “frustrating, but not surprising” given the relative lack of attention to health issues that affect marginalized groups.
Pulse oximeters, the fingertip devices used to non-invasively measure oxygen levels, have been known for decades to overestimate oxygen levels in patients with darker skin because melanin in the skin can interfere with the signals the devices read. Interest in the issue rose during the Covid-19 pandemic when the devices were used frequently to triage patients for emergency or ICU admission, and after a high profile 2020 study in the New England Journal of Medicine found Black patients were nearly three times more likely than white patients to have low oxygen levels go undetected. Subsequent studies have shown the devices led to delays in Covid treatment and supplemental oxygen use, and higher mortality rates for patients with darker skin.
Spurred by these reports, FDA officials have issued a warning about the devices, held advisory board meetings, written a discussion paper on the issue and said they would act to release new guidance for manufacturers to help create more equitable devices. FDA spokesperson Carly Pflaum said the agency was actively working to publish draft guidance “as expeditiously as possible.”
Delays in the publication of that guidance — promised last year, but now expected in fiscal year 2025 according to the FDA — have angered health equity leaders who say the inaction on the issue is endangering the lives of Black people and frustrated physicians who rely on the devices to assess oxygen levels for clinical decision making.
Iwashyna, who is working in the ICU this holiday, said he finds himself forced to decide if Black patients need painful arterial punctures to determine their true oxygen levels. “We have to make harder choices for our Black patients than for our white patients, because the pulse oximeters just are not as reliable in our Black patients,” he said.
advertisement
Some manufacturers, citing their own internal studies, have argued that their devices do work well on a range of skin tones. More research is underway to create more equitable devices and better guidance for testing them.
A related editorial in Monday’s issue of JAMA, which includes some of the same authors on the study, said fixing pulse oximeters is an example of a “wicked problem” — one with no easy solution that involves different decision makers whose values conflict. The editorial said stronger and clearer FDA guidance is needed, but may not be enough to usher in change. Challenges, they said, include the costs to manufacturers of devising more equitable devices and testing them on larger groups of more diverse subjects, and the fact that many manufacturers have been reluctant to admit their devices may be biased.
Several device manufacturers, including Medtronic, have settled a lawsuit brought by CEO Noha Aboelata of Roots Community Health, an Oakland, Calif.-based community health center, and have agreed to provide warnings to consumers and medical staff about their devices. Senators and the attorneys general of several states have called for change as well.
That change could be encouraged, the editorial said, if federal purchasing power provided pre-purchase agreements of more equitable devices now in development, or provided higher reimbursements for devices proven to be more equitable.
“Let’s say a less biased device comes on the market but is a lot more expensive,” said Carmel Shachar, an assistant clinical professor of law at Harvard Law School and first author on Monday’s editorial. “Without economic incentives, it may be difficult to get rapid adoption of the newer devices.”
Shachar said she also worried that new FDA guidance would do little to fix devices already in use. “Some concerns I have are whether this is going to apply only for new devices moving forward, and still allow the older devices to remain on the market, which it probably will,” she said.
advertisement
Another route to possible change now being discussed is through Section 1557 of the Affordable Care Act which prohibits discrimination within health care and would give patients who think they have been harmed by medical providers using known faulty devices the right to sue. One study showed that in the VA system alone, the devices accounted for 80,000 missed measurements of hypoxemia, or low oxygen, in a given year, numbers that may not change until better devices are put into use.
“More is needed to help develop and bring to market newer devices that don’t have the same problems,” said Shachar. “Once we have alternatives, more thinking needs to be done about whether we can remove the older devices from use.”
STAT’s coverage of health inequities is supported by a grant from the Commonwealth Fund. Our financial supporters are not involved in any decisions about our journalism.