What You Should Know:
– GE HealthCare (Nasdaq: GEHC) has received FDA 510(k) clearance for its innovative SIGNA™ MAGNUS, a 3.0T high-performance, head-only magnetic resonance imaging (MRI) scanner.
– This system offers new capabilities for both clinical imaging and neuroscience with the potential to aid in the detection of neurological, oncological, and psychiatric conditions.
SIGNA MAGNUS: Advancing Neuroimaging with Cutting-Edge MRI Technology
The FDA clearance of the SIGNA MAGNUS by GE HealthCare marks a significant leap forward in neuroimaging technology. The system incorporates a range of innovations designed to enhance imaging precision and research capabilities, making it a game-changer for clinical and research applications.
– Innovative Gradient Coil Design: SIGNA MAGNUS features an asymmetrical, high-efficiency, head-only gradient coil design. This design reduces the coil’s inner diameter, optimizing gradient performance specifically for neuroimaging. By shifting the gradient isocenter to the patient edge of the coil, the design provides easier patient head access and eliminates shoulder width constraints typically encountered with conventional MRI systems.
– Enhanced Gradient Performance: The head-only gradient coil achieves superior gradient amplitude and slew rates compared to conventional 60cm or 70cm bore whole-body MRI systems. The HyperG gradient technology, with its performance levels of 300 mT/m and 750 T/m/s, enables faster image acquisition using the same power requirement as the SIGNA Premier 3.0T system. This leads to improved spatial resolution, image clarity, and faster scans, particularly benefiting patients with difficulty remaining still or those who experience claustrophobia.
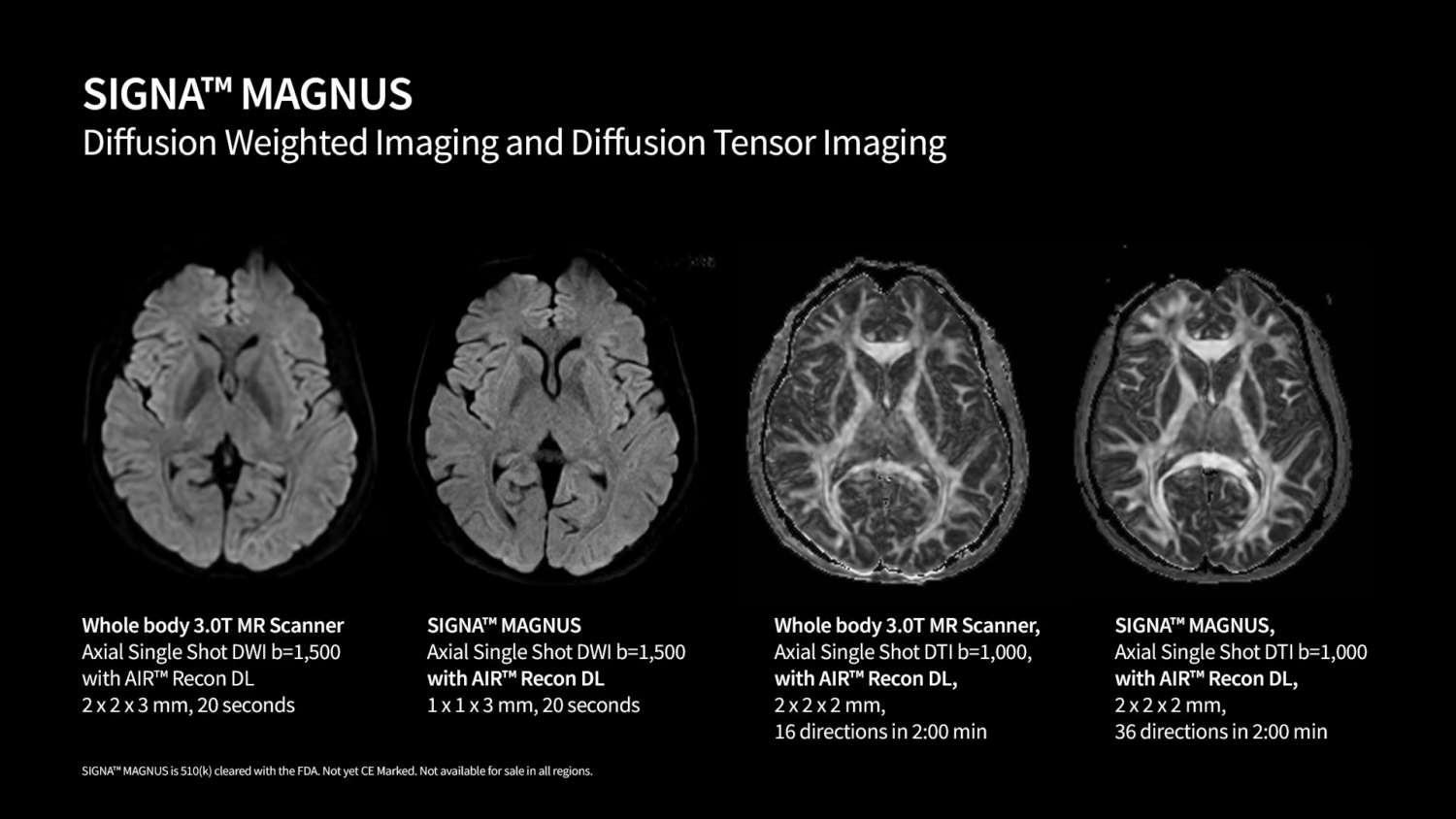
– Exceptional Imaging Precision: SIGNA MAGNUS provides high-resolution, high signal-to-noise ratio imaging with advanced diffusion techniques. The system enables short scan times while maintaining exceptional imaging quality, which is crucial for accurate diagnoses, particularly in neurodegenerative, neuro-oncology, and psychiatric disorders.
– Research Advancements: The system’s capabilities extend to advanced research applications, such as high B-value diffusion imaging, fMRI to investigate the BOLD (Blood Oxygen Level Dependent) response, and the measurement of slow Cerebral Spinal Fluid (CSF) flow. Its ability to visualize brain function, microstructure, and micro-vasculature through techniques like Oscillating Gradient Diffusion Encoding (ODEN) offers significant promise for neurological oncology research by providing critical cellularity contrast.
– Accessibility and Upgradability: SIGNA MAGNUS will be available for both forward production and upgrades from compatible SIGNA Premier systems, enabling existing facilities to integrate the advanced technology without the need for new systems, additional power, or cooling requirements. This flexibility enhances access to high-performance imaging and positions SIGNA MAGNUS as a transformative tool for both clinical practice and research.
– Clinical and Research Impacts: The availability of SIGNA MAGNUS empowers healthcare providers to make more accurate diagnoses, facilitating better treatment options. By advancing research in key areas such as neurodegenerative diseases, neuro-oncology, and psychiatric disorders, SIGNA MAGNUS is set to revolutionize neuroscience research and clinical care.
Overall, SIGNA MAGNUS represents a significant step forward in neuroimaging technology, combining advanced gradient performance with enhanced imaging capabilities to support both clinical practice and cutting-edge research.
“Obtaining FDA clearance further validates our commitment to not only innovating but also in delivering clinical technologies that have real-world impact,” said Jason Polzin, GM, MR Applications Platform and Research Technologies, GE HealthCare. “With SIGNA MAGNUS, we are providing neuroradiologists and neuroscience researchers a tool that supports advanced imaging and biomarker research and discovery previously impossible on conventional systems. It is our intent to make SIGNA MAGNUS widely available as a fully cleared commercial product.”

