Dive Brief:
- GE Healthcare has received 510(k) clearance for a tool designed to help clinicians assess people who may have Alzheimer’s disease, the company said Tuesday.
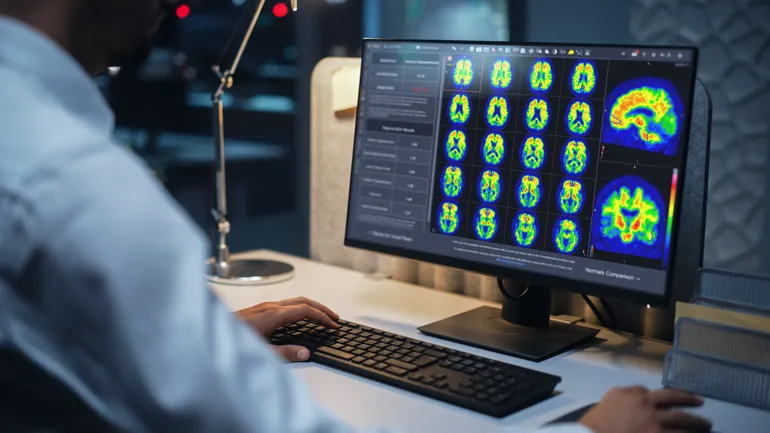
- The clearance adds a Centiloid scale tool to GE Healthcare’s Mim software to enable clinicians to determine the density of amyloid plaque in a patient’s brain from PET scans. Amyloid is a protein that, in Alzheimer’s, can clump up into toxic plaques in the brain. Scientists have linked the formation of those plaques to symptoms such as memory loss.
- Researchers developed the Centiloid scale, which Eisai and Eli Lilly used during the development of their recently approved Alzheimer’s drugs, to standardize amyloid imaging measures.
Dive Insight:
PET radiotracers facilitate the visualization of amyloid plaque density, enabling clinicians to assess people with cognitive impairment for Alzheimer’s and evaluate their response to treatment. However, different centers have reported different measures of PET tracer retention. The variability led to the development of the Centiloid scale in 2015.
The Centiloid scale is from zero to 100, with zero representing the average value from “high-certainty” patients who are negative for amyloid and 100 representing the average value from typical patients with Alzheimer’s, GE Healthcare said in the release.
The need for tools to evaluate amyloid via PET scans has increased as drugs that target the plaques have come to market in recent years. Last year, the Centers for Medicare and Medicaid Services changed its policy on PET scans, clearing a barrier to their use in the assessment of Alzheimer’s patients.
GE Healthcare acquired software to support amyloid imaging in its takeover of Mim Software in April. Now, the company is bringing a Centiloid scale to PET scanners with its vendor-neutral software, Mimneuro. The workflow generates standardized tracer uptake values for target regions of the brain and presents the data to clinicians in a report.
While GE Healthcare now owns Mimneuro, the software works with PET tracers from multiple suppliers. GE Healthcare’s Vizamyl is supported, as are Lilly’s Amyvid and Life Molecular Imaging’s Neuraceq. Sales of Vizamyl are currently “a few million dollars,” GE Healthcare CFO James Saccaro said on an earnings call in July, but the company expects revenues to grow as uptake of Alzheimer’s therapies increases.
GE Healthcare also sells PET scanners. Mimneuro provides GE Healthcare with further exposure to the Alzheimer’s growth opportunity and positions the company to generate sales even if clinicians use PET products from other vendors.