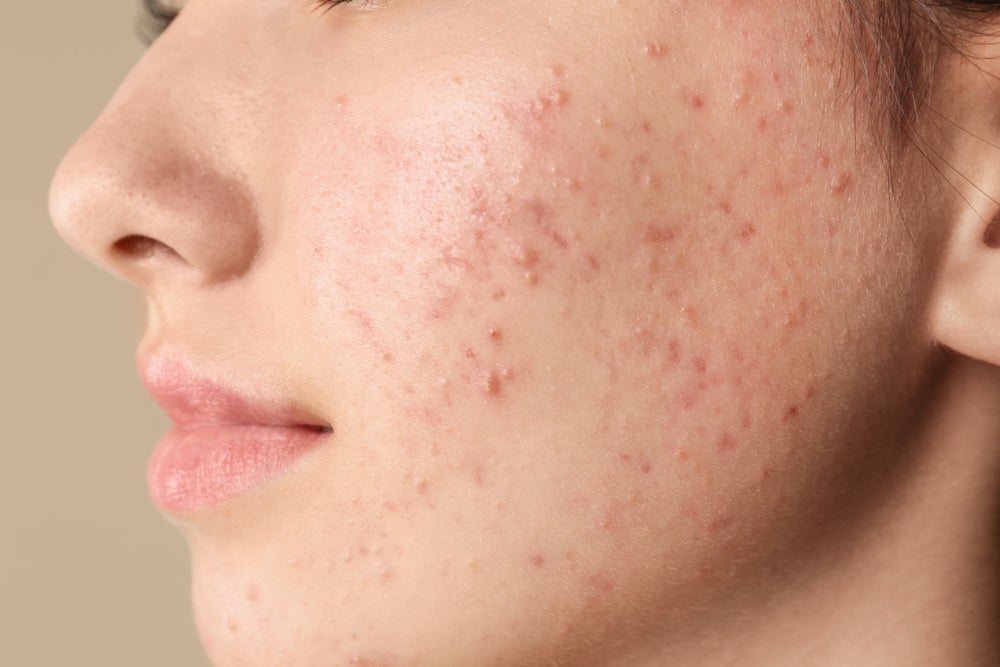
Glenmark has gained exclusive rights from Cosmo to commercialise the acne treatment Winlevi (clascoterone cream 1%) in Europe and South Africa, further expanding the reach of the treatment that is already popular in the US.
As per the deal, Glenmark will pay Cosmo’s subsidiary Cassiopea $5m to market Winlevi in 15 EU countries as well as the UK and South Africa. Cosmo, which will exclusively supply the drug, is also in line for further regulatory, royalty and sales milestone-based payments.
Glenmark now has rights to roll out the drug in countries including France, Spain, Portugal, Denmark, and the Netherlands. However, the company did not announce a timeline for when it expects to roll out the drug in all other EU countries, which also include Norway, Sweden, Finland, Iceland, Czech Republic, Hungary, Poland, Romania, Slovakia, and Bulgaria.
Glenmark stated it will register Winlevi in the UK and South Africa, whereas Cassiopea will be responsible for Europe – overseeing the European Medicines Agency’s (EMA) centralised marketing authorisation.
Winlevi, approved by the US Food and Drug Administration (FDA) in 2020, has been a popular treatment for acne in the US. In end-of-year financial results revealed in March 2023, Cosmo stated Winlevi is the most prescribed topical acne product in the US. It generated sales of $28m (€26.7m) in 2022. GlobalData predicts the drug will generate US sales of $257m by 2029, with availability in Europe expected to drive this even further.
GlobalData is the parent company of Pharmaceutical Technology.
Winlevi is a topical androgen receptor inhibitor. According to Glenmark, the drug’s non-antibiotic approach fills an unmet need in Europe – where guidelines currently discourage the use of topical antibiotics for acne due to risk of resistance.
Cosmo has already partnered with Hyphens Pharma for the drug’s commercialisation in Southeast Asia, with Hyundai Pharmaceutical in South Korea, and 3SBio in China.
Professor Alison Layton, associate medical director for research, Harrogate and District NHS Foundation Trust said in a statement accompanying the deal: “[Winlevi] could be prescribed as monotherapy or as part of an acne regime including fixed topical combinations and systemic antibiotics. All patient populations with acne could potentially receive and benefit from clascoterone.”
