
The world of clinical trials is undergoing a rapid change. Today, the rise of decentralized clinical trials (DCTs) and hybrid models has transformed the landscape, offering a more flexible, patient-centric approach that is breaking down traditional barriers. With advances in technology — particularly in mobile health apps, telemedicine, and wearable devices — clinical research is becoming more efficient, accessible, and inclusive.
GlobalData’s Clinical Trials Database is currently tracking 16,076 decentralized clinical trials around the world at various stages, from planned to completed. This includes 9,291 drugs and 9,108 companies.
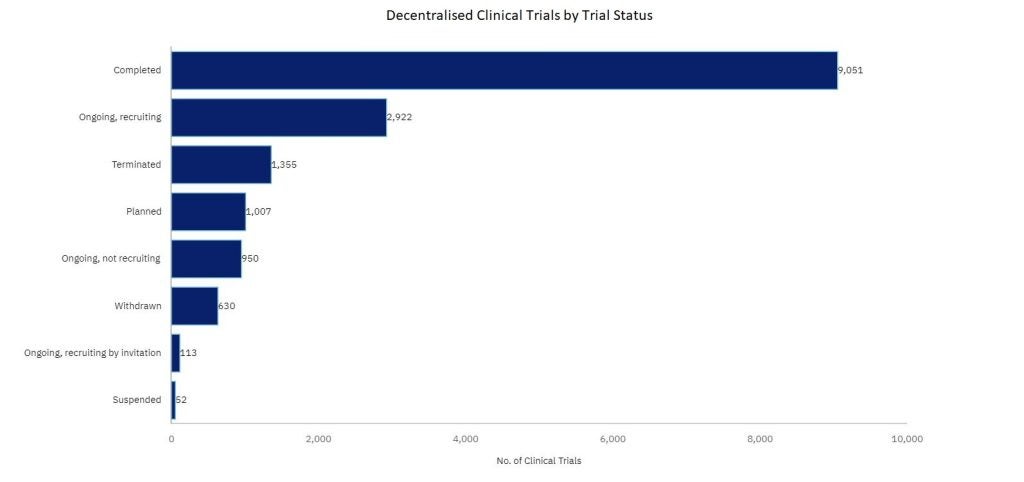
The growing adoption of decentralized clinical trials
According to GlobalData’s clinical trial database, the adoption rate of decentralized clinical trials was particularly pronounced following the disruptions caused by the COVID-19 pandemic, which accelerated the need for trials to proceed without traditional site-based constraints. During this period, DCTs provided a crucial means of ensuring continuity, allowing patient recruitment and data collection to continue remotely. As a result, many sponsors have now realized that decentralized approaches can increase efficiency, reduce costs, and provide access to more diverse participant pools.
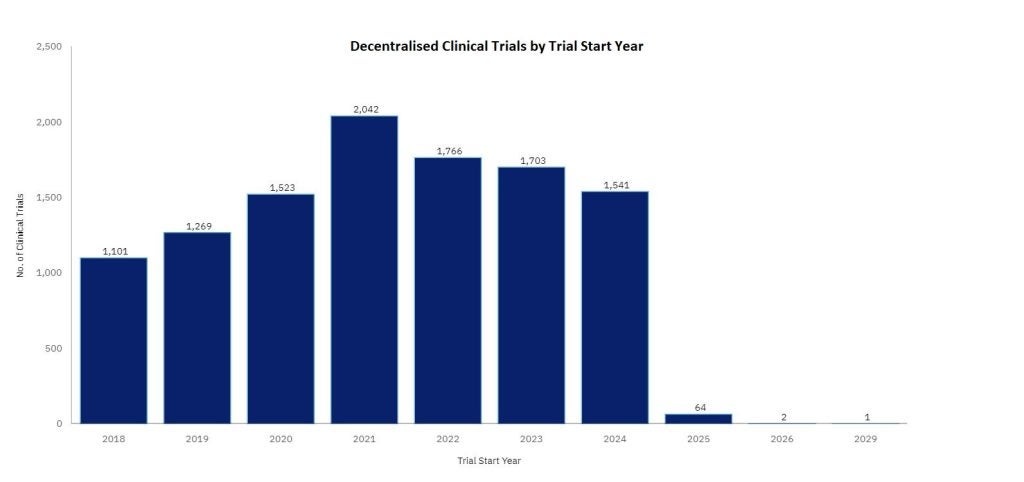
Global reach: expanding patient access
One of the most significant advantages of DCTs is their ability to expand the geographical and demographic reach of clinical trials. Traditionally, site-based trials have been limited to participants who live within a reasonable distance of the trial site. This has often resulted in underrepresentation of certain populations, particularly those in rural or underserved areas.
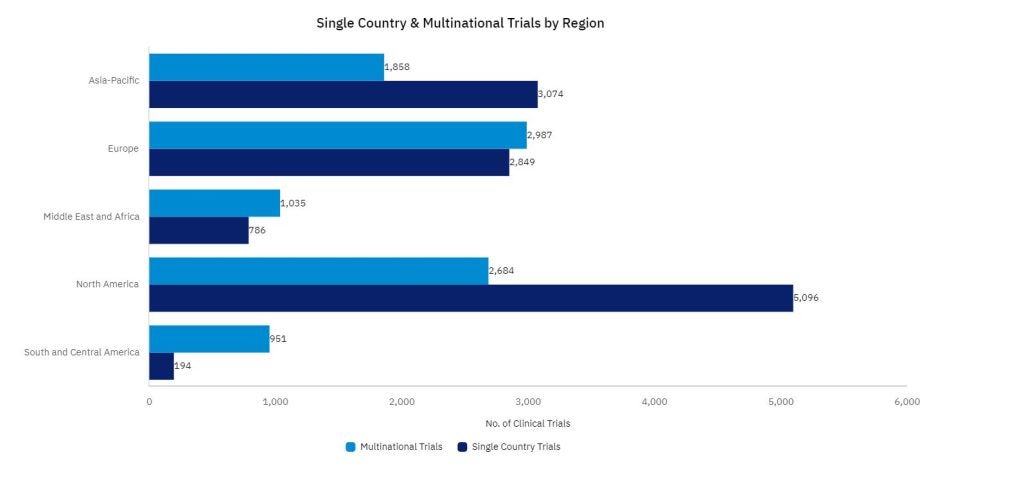
The geographical reach of decentralized trials has widened significantly, with multinational trials becoming more common. GlobalData’s data shows a clear increase in the number of decentralized trials that span multiple countries, reflecting the ability of DCTs to tap into more diverse participant pools. This is particularly important for trials studying conditions that disproportionately affect certain populations or that require a broad demographic to generate comprehensive results.
The DCT market has a global reach. North America continues to move towards increased use of DCT trials, as big pharmaceutical sponsors based in the US are looking for new ways to streamline clinical trials, and DCTs have been proven to be effective in doing so.
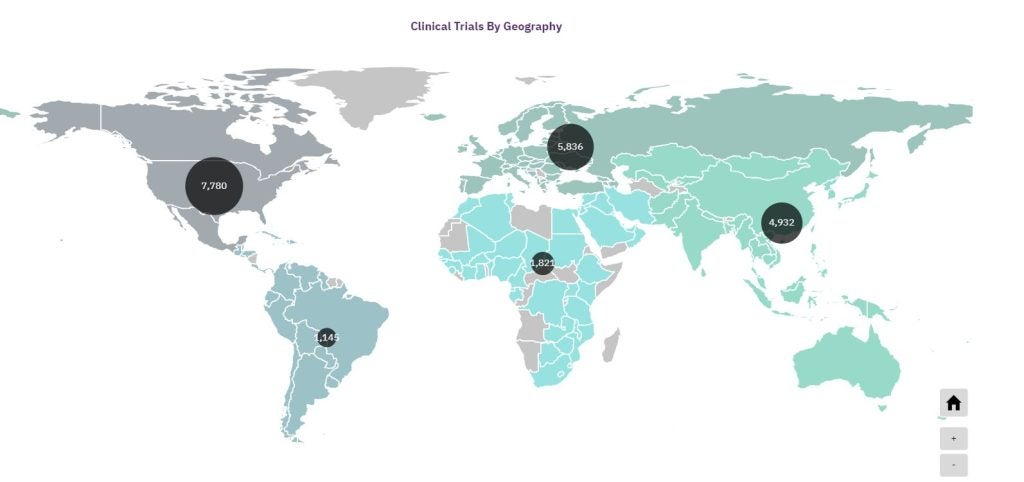
By removing the need for participants to travel to trial sites, DCTs also make it easier for those with limited mobility or who cannot take time off work to participate. This not only increases the inclusivity of trials but also accelerates recruitment times, as participants are more likely to enroll if the process is convenient.
Key mobile components in decentralized trials
The success of DCTs and hybrid models largely hinges on the use of mobile components, such as apps, telemedicine platforms, and wearables. These tools are essential for collecting real-time data, monitoring patient health, and ensuring continuous engagement throughout the trial period. The use of mobile components has steadily increased in trials over the past several years, according to GlobalData’s database.
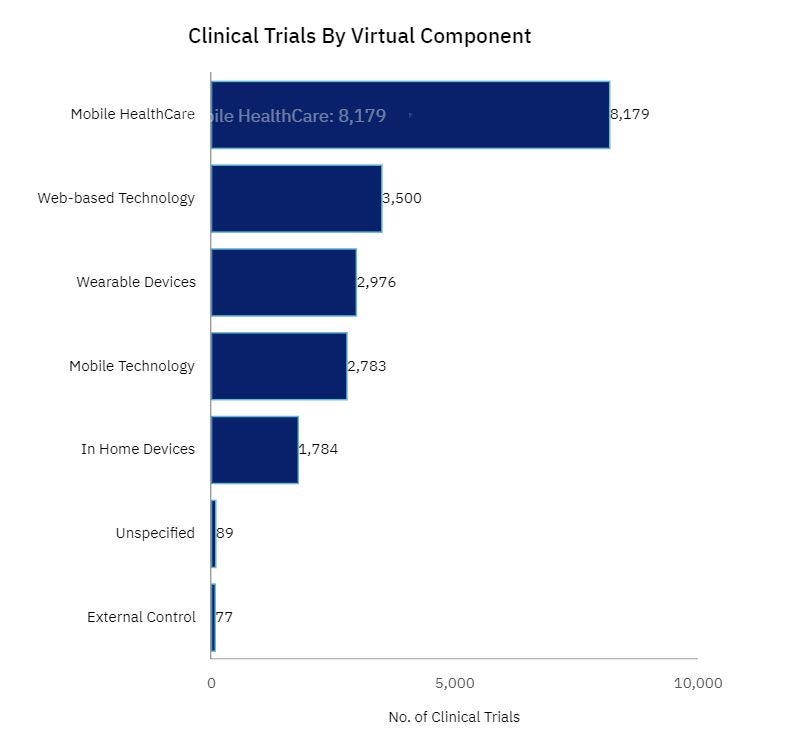
Mobile applications are a key element, providing participants with an easy way to log their daily health metrics, complete surveys, and report any adverse events. Wearables are proving to be invaluable for trials that require continuous monitoring of vital signs – such as heart rate, blood pressure, and glucose levels. These devices allow for the remote collection of high-quality data that would otherwise require frequent clinic visits.
Telemedicine is also playing a pivotal role in the decentralized trial model. By enabling virtual consultations, telemedicine reduces the need for in-person visits, making trials more convenient for participants. It also allows for more frequent interactions between patients and study coordinators, improving oversight and addressing any concerns participants may have in real time.
Hybrid models: The best of both worlds
While fully decentralized trials are gaining popularity, hybrid clinical trials are emerging as a preferred option for many sponsors. These trials combine decentralized methods, such as remote monitoring and virtual patient engagement, with in-person visits for procedures that cannot be done remotely. The hybrid model’s flexibility allows for the inclusion of traditional, site-based elements when necessary, while still taking advantage of the efficiencies offered by decentralized approaches.
Incorporating elements such as mobile apps, telemedicine consultations, and wearable devices allow for continuous patient monitoring, significantly reducing the need for in-person visits while maintaining data integrity and patient safety. This balance between remote and on-site engagement is particularly valuable for complex trials requiring specialist procedures that can only be performed in a clinical setting.
The hybrid approach also addresses one of the most significant challenges in DCTs: ensuring patient compliance and high-quality data collection. By integrating decentralized technologies with traditional methods, sponsors can offer a more personalized and flexible experience for participants. This reduces drop-out rates and improves patient retention, leading to more reliable trial outcomes.
The role of technology in driving hybrid decentralization
At the heart of the decentralization movement is the widespread adoption of advanced digital technologies. Mobile apps, wearable devices, telemedicine, and electronic patient-reported outcomes (ePROs) have become indispensable tools for conducting DCTs and hybrid trials. These technologies not only make it easier for participants to engage in trials but also enhance the quality and timeliness of the data collected.
Datacubed is one of the companies leading the charge in this digital transformation. By providing an intuitive platform that integrates seamlessly with mobile technologies, Datacubed helps sponsors design and manage decentralized and hybrid trials more effectively. Their platform enables real-time data collection, patient monitoring, and remote engagement, all of which are crucial for the success of modern clinical trials. By utilizing remote monitoring, Datacubed helps clinical trials collect patient data more frequently and in a timely manner, monitor and track patient progress, and make adjustments in real-time.
Datacubed’s platform addresses several key challenges faced by hybrid clinical trials, such as maintaining data integrity and ensuring patient compliance. Its DCT platform has proven to deliver 90% compliance and 80% patient retention, incorporating technology such as eCOA/ePRO, eConsent, geofencing, and televisits.
Through mobile apps, participants can easily log their symptoms, complete questionnaires, and even communicate with study coordinators. The platform’s integration with wearable devices allows for continuous monitoring of vital signs, providing researchers with real-time insights into a participant’s health status. This real-time data collection is critical for making timely decisions about patient care and trial adjustments.
Overcoming regulatory and compliance challenges
As decentralized trials become more prevalent, sponsors must navigate a complex regulatory landscape. Each country involved in a multinational trial may have different regulations regarding patient privacy, data sharing, and clinical oversight. Moreover, the use of digital platforms introduces new challenges related to data security and compliance with global standards such as the General Data Protection Regulation (GDPR), and the Health Insurance Portability and Accountability Act (HIPAA).
Datacubed has been proactive in addressing these regulatory challenges, building robust compliance features into its platform. The company works closely with regulatory bodies to ensure that its systems meet the stringent requirements of various jurisdictions. This not only helps to safeguard patient data but also ensures that trials remain compliant, regardless of where participants are located.
The future of clinical trials is hybrid
The rise of decentralized and hybrid clinical trials represents a fundamental shift in the way medical research is conducted. As the data from GlobalData illustrates, these models are not just a passing trend — they are becoming a cornerstone of modern clinical research.
According to a GlobalData survey, patient recruitment and participation are the primary incentives for adopting decentralized clinical trials in the future. While many respondents used DCTs pre-pandemic – mostly in North America and Europe – 37% now plan to use DCTs specifically having seen the benefits. In addition, many more stated that DCTs are already planned, the pandemic just sped up their adoption. A total of 74% of respondents believed DCTs will be most frequently used in the next one to four years.
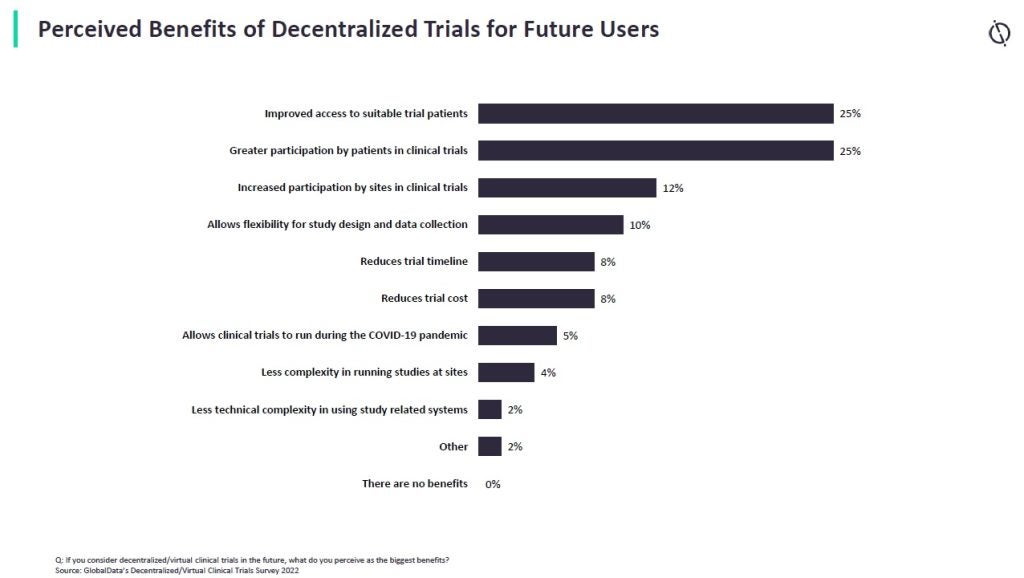
Looking ahead, the continued growth of decentralized and hybrid models will likely reshape the clinical trials landscape even further. With the adoption of new technologies and the growing acceptance of remote engagement, clinical trials will become more inclusive, efficient, and patient-centric. As more sponsors embrace these models, the industry will continue to evolve, bringing life-saving treatments to market faster and more effectively than ever before.
To find out more, download the whitepaper below.

