Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good summer morning! Today, we discuss the big Medicare price negotiations (and the not-entirely-meaningful numbers the White House is touting), see a 75% personnel cut at Lykos Therapeutics, and more.
advertisement
By the way: Elaine and I are taking next week off, so we’ll see you bright and early on Monday, Aug. 26!
The need-to-know this morning
- Pfizer and BioNTech said their combined mRNA vaccine candidate against influenza and Covid-19 showed a lower immune response against one type of influenza, influenza B, in a Phase 3 trial, a setback for the vaccine.
- In an interview with STAT, Bavarian Nordic CEO Paul Chaplin said his company could manufacture and distribute an additional 2 million doses of mpox vaccine by the end of the year, and another 8 million doses in 2025.
White House over-inflates Medicare price reductions
Thanks to its new Medicare negotiation process, the White House has been flashing year-over-year drug price reductions about — but the numbers leave a false impression. Medicare doesn’t typically pay full list prices because smaller-scale (and confidential) discounts have already been negotiated by prescription drug plans.
Across the 10 drugs selected for Medicare negotiations, the real impact is a more modest 22% reduction in prices compared to last year, as opposed to 40%-80% based on list prices.
advertisement
Lykos cuts 75% of staff, Rick Doblin leaves
Lykos Therapeutics, whose MDMA-assisted psychotherapy for PTSD was recently rejected by the FDA, is reorganizing: It has laid of 75% of its staff, and Rick Doblin — —a pioneer in psychedelia — has stepped down from the company’s board. Lykos plans to focus on clinical development and working with the FDA to address its concerns, and move forward with a new drug application.
Doblin will focus his efforts on his nonprofit MAPS and continue broader efforts in psychedelic research and policy reform.
“After 38 plus years of work, I’m profoundly saddened by the FDA decision around this critically needed therapy, but am heartened that Lykos will still move forward continuing clinical research that addresses the FDA’s questions,” he said in a statement.
There’s too much lab space in Boston
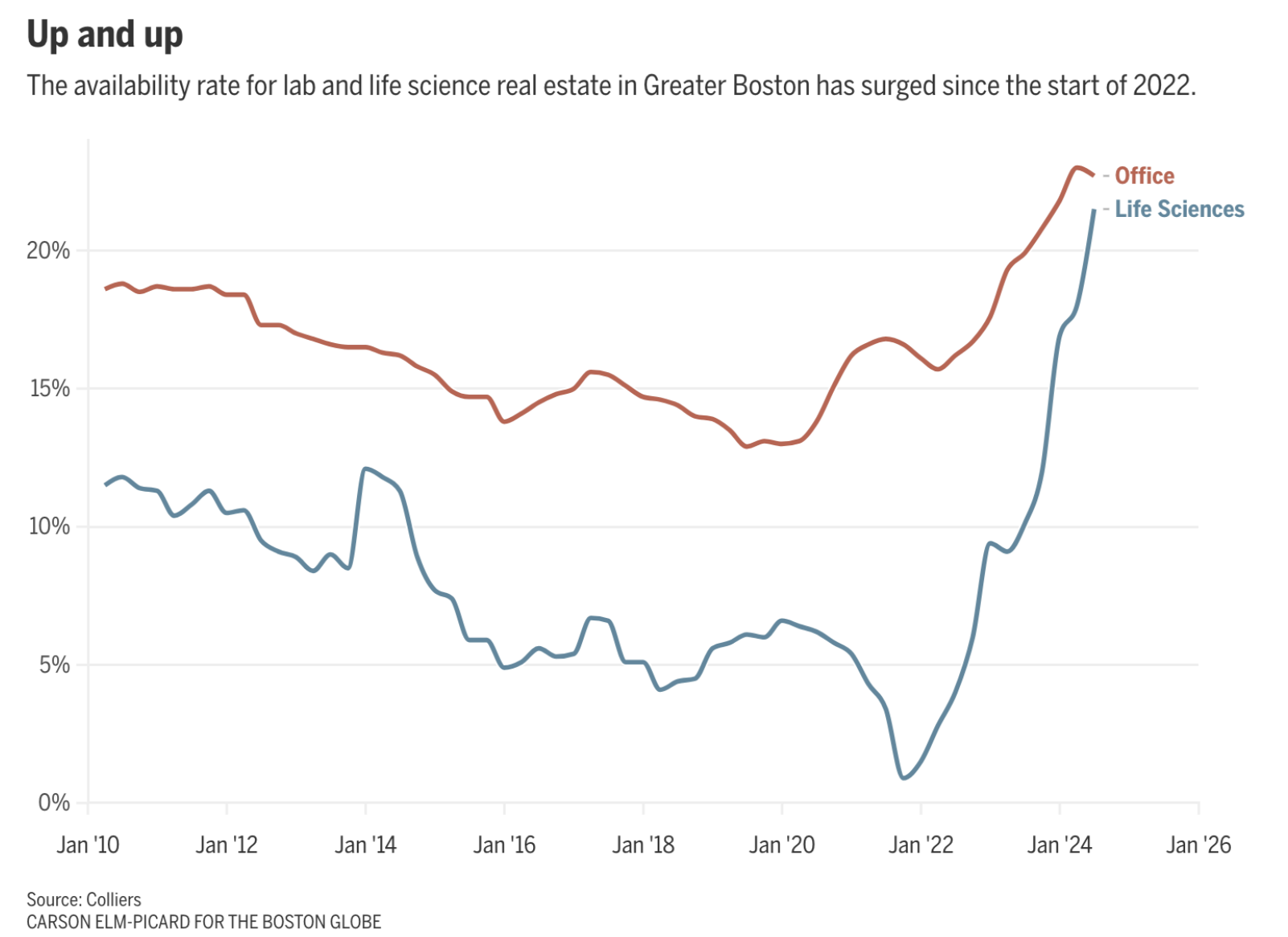
Demand for lab space during the Covid-19 pandemic spurred rapid real estate development in the Boston area — but now there’s a tremendous oversupply. Right now, 21.5% of lab space there is vacant, compared to just 1% three years ago. This surge in vacancies is attributed to speculative building by developers, an influx of venture capital during the pandemic, and the broader slowdown of the biotech sector.
This amounts to about 11 million square feet vacant in the Boston area, with more expected as new projects are completed. While some look at this as a market correction, others actually look at it as a chance for smaller biotechs to grow.
Is pharma still fearful of the Inflation Reduction Act?
Will Lykos’ FDA rejection and recent publication retractions pull down the entire psychedelics industry? And what would you name a commercial psychotherapy treatment? We discuss all that and more on this week’s episode of “The Readout LOUD.”
STAT’s chief Washington correspondent Rachel Cohrs Zhang joins us to discuss the Medicare drug price discounts and how this first round of negotiations between pharmaceutical companies and Medicare officials played out. After that, I join Elaine, Allison, and Adam to talk about some of the latest news in the psychedelics world, including the retraction of three research papers.
Gilead’s patent hopping with unsafe HIV drug
Gilead Sciences, which is a top HIV drugmaker, settled a $40 million lawsuit from 2,625 people who claimed they were harmed by the drug tenofovir disoproxil fumarate, or TDF. And a larger case in California, which involves 24,000 plaintiffs, accuses Gilead of delaying the release of a safer version of of the drug — saying the company is maximizing profits by patent hopping, or switching patients to the new drug only right before TDF’s patents expire, despite knowing it’s unsafe.
“When pharmaceutical companies do good work, they should be able to turn a decent profit,” opines Peter Staley, cofounder of PrEP4All, an advocacy organization promoting a national HIV prevention plan. “But dragging their feet to bring a safer treatment to market while thousands of people are taking a harmful one isn’t doing good work.”
More reads
- Genentech dissolves cancer immunology group, Endpoints News
- ‘We are firing on all cylinders’: Bayer pharma BD chief says group overhaul not slowing dealmaking, FierceBiotech
- Vaccine group Gavi says it has up to $500 million for shots, Reuters
- AstraZeneca’s Imfinzi wins FDA priority review for limited-stage small cell lung cancer, MarketWatch

