Last month, Vertex Pharmaceuticals and CRISPR Therapeutics made history, by gaining approval for the first clustered regularly interspaced short palindromic repeats (CRISPR)-based drug, exagamglogene autotemcel (Casgevy).
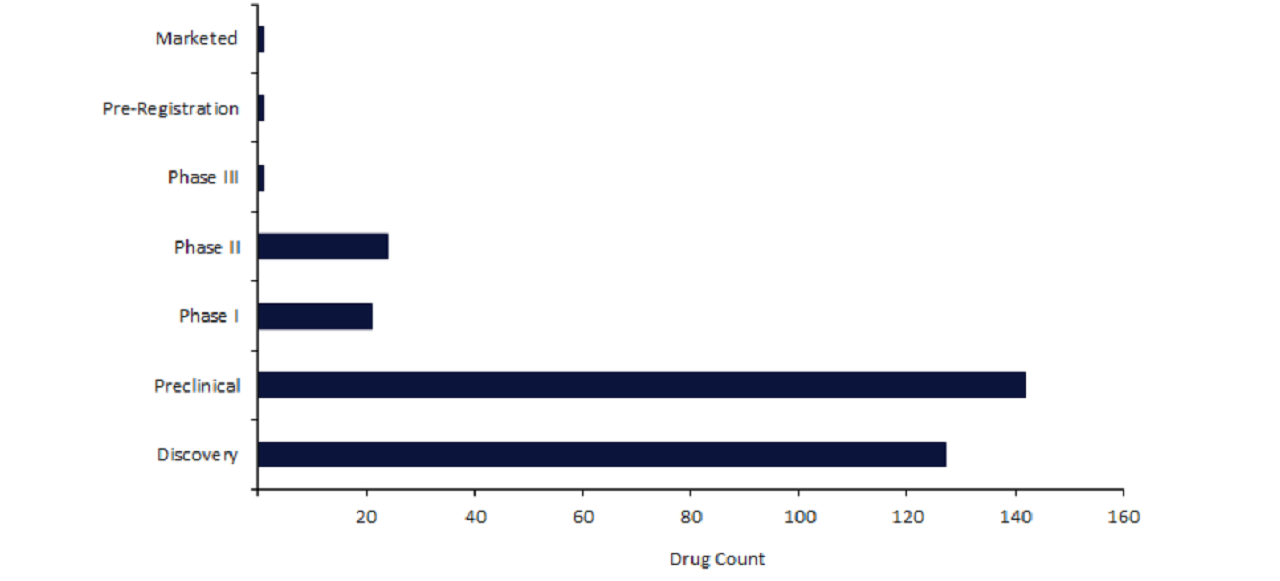
However, given that 88% of CRISPR drugs are in the early stages of development, it is unlikely that we will see another CRISPR approval any time soon.
On 15 November, Casgevy secured the first approval for a CRISPR-based drug from the UK’s Medicines and Healthcare products Regulatory Agency for the treatment of sickle cell disease and beta thalassemia.
This groundbreaking decision made history, by approving the first genome-editing drug. The approval was swiftly followed by the US Food and Drug Administration’s approval for sickle cell disease on 8 December.
This revolutionary treatment offers a potential cure for inherited blood disorders, which are caused by errors in the haemoglobin genes.
Previously, the only permanent treatment option for the blood conditions was a bone marrow transplant, which carries a plethora of risks, including rejection.
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalData
Casgevy was developed following a 2015 partnership between Vertex Pharmaceuticals and CRISPR Therapeutics and works by precisely editing the faulty gene in a patient’s bone marrow stem cell, enabling the production of functional haemoglobin.
Currently, Casgevy is the only marketed CRISPR drug, as well as the only CRISPR drug featuring in preregistration and Phase III stages of development.
It is in preregistration for sickle cell disease in the EU, as well as beta thalassemia in the EU and US. Additionally, Casgevy is in Phase III for these indications in Canada.
However, despite the landmark approval of Casgevy, this feat is unlikely to be followed up any time soon by another CRISPR drug, given the current landscape of pipeline products.
Despite a gradual increase in CRISPR drugs from Phase II compared to Phase I, the earlier stages of development, discovery and preclinical, account for 88% of active CRISPR pipeline drugs.
This composition indicates that CRISPR drugs are still very much in their infancy and are yet to be consistently established throughout the later stages of drug development.
Nevertheless, there are currently 24 CRISPR drugs in Phase II, which includes CRISPR Therapeutics’s CTX-110.
If successful, GlobalData’s Catalyst Calendar predicts this drug to be the next CRISPR therapy to be globally launched, towards the end of 2025.
This drug is in Phase II for oncological indications such as B-cell acute lymphocytic leukaemia.
However, according to GlobalData’s Likelihood of Approval tool, it is predicted that this drug only has a 48% chance of advancing into Phase III trials, with only an overall 31% likelihood of approval.
The Casgevy approvals represent a significant milestone for revolutionary genome editing systems.
However, given the relative immaturity of the CRISPR drugs pipeline, which features very few late-stage products alongside a low likelihood of approvals, it is unlikely that we will see another drug approval any time soon.
It will be interesting to see how the success of Casgevy impacts CRISPR research and drug development, as time progresses.
