Dive Brief:
- Johnson & Johnson received Food and Drug Administration approval for its Varipulse pulsed field ablation (PFA) system, joining Boston Scientific and Medtronic in the U.S. market for the fast-growing atrial fibrillation (AFib) treatment.
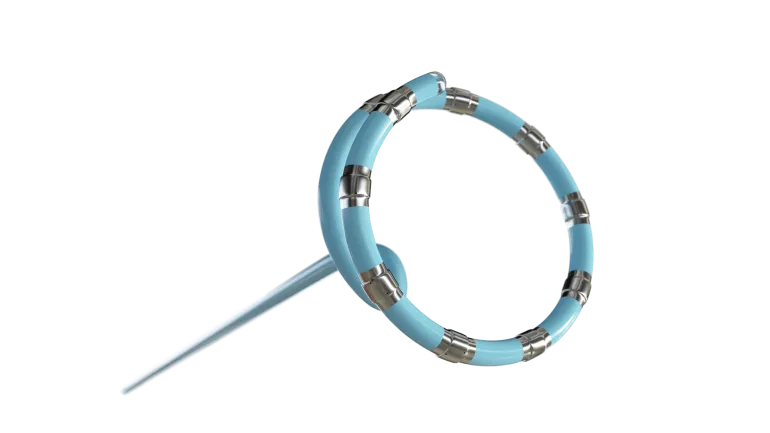
- Varipulse is approved to treat patients with drug-resistant, recurrent paroxysmal AFib, a common form of abnormal rhythm in the heart’s upper chambers, the company said Thursday. The platform is integrated with J&J’s Carto 3 mapping system in a single device that enables electrophysiologists to view inside the heart to position the catheter.
- “We are confident that this eagerly awaited platform will be a valuable tool for physicians in performing safe, effective and efficient AFib procedures with an intuitive and reproducible workflow,” Jasmina Brooks, president of electrophysiology at J&J Medtech, said in a statement.
Dive Insight:
Physicians have quickly adopted PFA for AFib treatment because they view it as a safer and more efficient ablation technology. Older methods use radiofrequency energy or extreme cold to create small scars to block AFib’s irregular heartbeats, whereas PFA ablates cardiac tissue with short high-voltage pulses.
J&J’s Varipulse, which is also approved for use in Europe, Japan and Canada, will now compete with Boston Scientific’s Farapulse and Medtronic’s Pulseselect PFA systems, both of which began rolling out in the U.S. this year. Medtronic also recently received FDA approval for its Affera mapping and ablation system to treat persistent AFib, and Boston Scientific gained FDA’s nod for Farawave Nav that enables mapping and PFA with a single catheter.
Boston Scientific last month said it expects PFA to exceed the company’s earlier projection for the procedure to comprise 40% to 60% of AFib ablations worldwide by 2026.
J&J anticipates the integration of its mapping system, which it said leads the industry in the category, will help it gain support for Varipulse in the U.S. market. Varipulse is the only PFA system in the U.S. to be fully integrated with Carto 3 mapping, the company said.
J&J Medtech Chairman Tim Schmid told analysts on an October earnings call that the company’s $5 billion electrophysiology business grew 11% in the third quarter from a year ago, with its mapping system used in more than 50% of competitive cases.