Dive Brief:
- The Food and Drug Administration has sent a warning letter to Johnson & Johnson’s Abiomed business, citing failure to report quality problems with the company’s Impella heart pump and lack of premarket approval for a monitoring system used with the device.
- In the Sept. 19 letter, the regulator identified several quality management procedures and medical device reporting requirements it said the company failed to follow leading up to a recall earlier this year of a subset of Impella 5.5 heart pumps. In addition, an FDA analysis found certain features of a monitoring system for the Impella pump are software device functions that require premarket authorization. The FDA posted the warning letter to its website on Tuesday.
- Impella heart pumps remain on the market and available for patient use, a J&J spokesperson said Wednesday in an emailed statement. “We are working closely with the FDA to fully resolve the observations as quickly as possible. As we continue to integrate into Johnson & Johnson MedTech, we are implementing quality system process improvements related to the observations, and many of these corrective actions are already in process,” the spokesperson said.
Dive Insight:
J&J completed the $16.6 billion acquisition of Abiomed in December, accelerating a push into faster-growth markets. It was the medtech sector’s largest deal of 2022. Since then, the company has been working to integrate Abiomed into its cardiovascular portfolio, which it retained as part of Johnson & Johnson MedTech after spinning off its consumer health arm.
At the same time, Abiomed has faced complaints about fluid leaks that could cause the pump to stop working and damage to the purge sidearm in some Impella 5.5 devices.
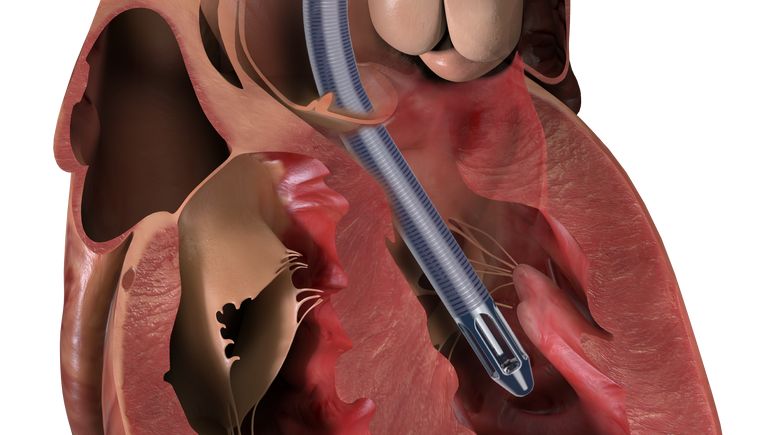
The Impella device supports the heart’s pumping chambers in cases of cardiogenic shock after a heart attack or open heart surgery, or when the heart muscle is not functioning efficiently due to a condition called cardiomyopathy.
Abiomed initiated a recall for 466 Impella 5.5 with SmartAssist devices in April, reporting 179 complaints, three injuries and no deaths tied to the implant, which is designed to take over pumping duties for the heart on a short-term basis.
In its warning letter, the FDA said that despite an “unacceptable elevated rate” of complaints, Abiomed initiated no correction or recall until the agency conducted its most recent inspection at the company on March 1 through April 13.
The letter also faulted Abiomed for failing to file timely medical device reports with information about malfunctions that may have contributed to a death or serious injury. Abiomed in August said a retrospective review of 254 complaint records found “51 records were identified as requiring a product history review,” FDA noted.
“You conclude the 51 product history reviews conducted did not require additional actions or escalation,” the agency said, adding that no records were provided to verify this.
Abiomed on Wednesday said it has provided detailed instructions to customers on how to return and replace the recalled devices. The latest versions of the Impella 5.5 with SmartAssist sets, which have a preinstalled sidearm retainer and new yellow luer, were not part of the recall, the spokesperson added.
The regulator also took aim at a pump monitoring system, called Impella Connect, that lacks premarket approval or an investigational device exemption. Features of the monitoring system are “software device functions that require premarket authorization,” the agency said. The system allows users to remotely monitor the performance of pumps, view case information and filter notifications by alarm status.
“The FDA will evaluate the information that your firm submits and decide whether the product may be legally marketed,” the warning letter said.
The Impella Connect app does not affect the function of the pump, and it also remains on the market, J&J said. “The guidelines surrounding software device functions are evolving, and we are committed to working with the agency to resolve their observations,” the J&J spokesperson said.
The FDA warning letter does not address a second recall of Impella devices prompted by problems with how the pump interacts with transcatheter aortic valve replacement stents. Nearly 7,900 heart pumps were recalled in June due to the issue, which was linked to four deaths. Those devices remain in the field.