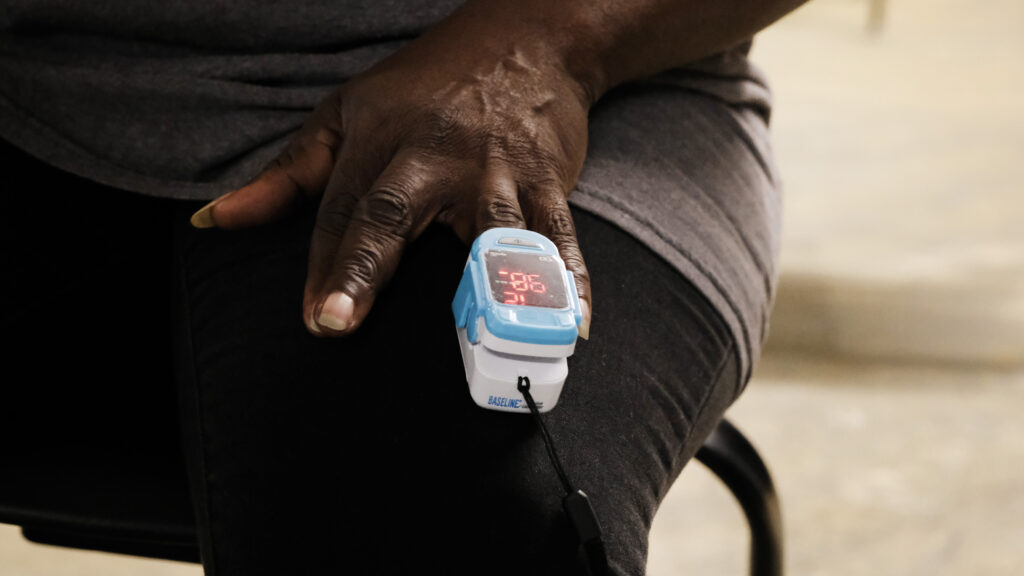
In 2022, Noha Aboelata noticed something troubling. The founding CEO of a health clinic that serves a predominantly Black and brown population, she realized that the racial biases built in pulse oximeters were hurting her patients in Oakland, California. The devices overestimate blood oxygen in people with darker skin tones, making them appear healthier than they are.
So she decided to pressure companies to make a more equitable device by suing 12 pulse oximeter manufacturers and distributors.
advertisement
That suit, filed almost a year ago, is beginning to create change. Several companies, including Medtronic, have settled. While companies haven’t disclosed the exact terms of the settlements, Medtronic has begun educating physicians about the shortcomings of its devices and providing QR codes on its devices that link to educational information on pulse oximetry.
“It’s definitely a step in the right direction — labeling is really needed here — but I think it just doesn’t go far enough,” said Sara Gerke, a professor of law at the University of Illinois College of Law. “We need to design better products that work for everyone.” To that end, Medtronic has also opened up a lab in Colorado with the goal of testing future devices on a more diverse set of skin tones.
Pulse oximeters, which measure levels of blood oxygenation by shining a light through a patient’s skin, have long been documented to be less accurate in more darkly pigmented skin. But the public became far more aware of their limitations during the height of the pandemic, when they made it harder for health care workers to assess the severity of Black patients’ Covid-19 cases. That, coupled with a desire to reckon with medicine’s racial inequities, put their failures in the spotlight.
advertisement
The lawsuit alone can’t fix the problems with pulse oximeters. Several senators urged the FDA to address the issue, and in 2022 the agency held a public meeting calling for clearer labeling and more rigorous testing of the devices. The following year, more than two dozen state attorneys general redoubled the call to make changes to the devices, citing STAT reporting.
Today, those who rely on the devices in emergency rooms and ICUs are still waiting on the FDA and are angered by the delay. “I should have had a better device by now,” Theodore J. Iwashyna, a pulmonary and critical care physician at Johns Hopkins who has researched the effect of the devices on darker-skinned patients since 2020, told STAT recently. “How many more of my patients need to die before we fix this?”
Aboelata, a family physician, is also frustrated with the pace of change. She was part of a study of a nearby health system that when pulse oximeters overestimated the blood oxygen in Black patients with Covid, they waited longer to be seen by a doctor and receive treatment. Her clinic’s lawsuit accused manufacturers and distributors of the device of violating California consumer protection law, arguing that companies were falsely advertising their devices’ efficacy.
Several other companies that distribute and sell oximeters, including Veridian Healthcare, Gurin Products, and Zewa have settled with Roots Community Health, Aboelata’s clinic. Medtronic is the first company that sells oximeters specifically to hospitals to settle in the suit. CVS Pharmacy, Walgreen, Compass Health Brands, Contex Medical Systems USA, Einstein Associates, ChoiceMMed America, Masimo, Covidien Sales, and GE Healthcare have yet to settle.
“We are pleased that Medtronic, which holds a dominant share in the pulse oximeter market, has agreed to prioritize patient safety by providing labels and brochures alerting their California hospital customers of the potential flawed readings for people with darker skin pigmentation,” Aboelata said in a statement.
advertisement
Medtronic said in a statement that it is “pleased to announce that Roots Community Health has agreed to dismiss claims against the company related to pulse oximeters. Medtronic looks forward to working with Roots, the FDA and other key stakeholders to ensure health equity can be achieved through technology, educational efforts, and partnerships.”
While the lawsuit would be applicable only in California, changes made in the state are often adopted nationally because of its large market share.
Now, Roots and other experts STAT spoke to are waiting for an update to FDA guidance, which they hope will lead to devices that work well on all skin tones. “There’s something problematic about a system that has a good tool for lighter-skinned patients and a lousy tool for darker-skinned patients,” Carmel Shachar, a professor of law at Harvard Law School says. “So education will help raise awareness of this problem, but it won’t necessarily help a physician figure out, ‘What do I do with my darker-skinned patient?’ That’s where the FDA guidance would come into place.”
Thomas Valley, a pulmonologist at the University of Michigan who has studied the issues with oximeters, noted that the FDA has put out a warning about the inaccuracies of the device. “One could argue it wasn’t that effective in that we continue to have problems,” he said. “A broad warning doesn’t necessarily trickle down to all the people who really need to know about it.” He also pointed out that several of the “major players” have yet to settle.
The FDA was slated to update its guidelines for devices by Sept. 30 but missed that target date.
The agency has said that it is “working diligently” to issue the new guidance, which it emphasizes offers best-practice recommendations for drug device manufacturers, not requirements.
“I am pleased with any progress, but ultimately the machines need to be fixed,” Aboelata told STAT. “It really is just a small piece of it. I do hope it’s a catalyst. I do hope it helps raise more awareness across the board, but I think we’re still a really long way from any justice on this issue.”
advertisement
This story has been updated to clarify that the label Medtronic added to its devices is a QR code leading to educational materials on pulse oximeters.