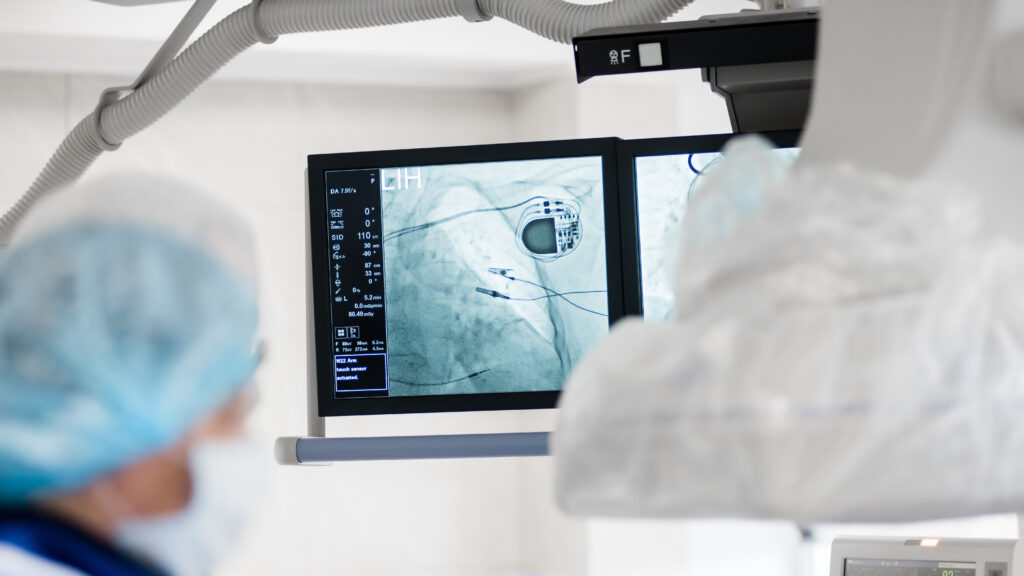
In her final presentation for health policy class at the University of Chicago, first-year medical student Robin Ji informed her classmates that the Food and Drug Administration does not require randomized controlled trials of most medical devices. Her peers’ immediate reaction was disbelief.
“One classmate kept asking me, are you sure?” Ji said. “I think he asked me twice. Then he went on his phone to check to see if it was actually true.”
advertisement
Ji was sure. She had spent two years before going to medical school working as a policy analyst for Rita Redberg and Sanket Dhruva, medical safety experts at the University of California, San Francisco. In a recent national survey published by the UCSF team in Health Affairs, Ji learned that it’s not just her classmates who may have misconceptions about the FDA — only 17% of physicians surveyed felt they understood the FDA’s device approval process. Around 41% felt they understood the drug side.
Get unlimited access to award-winning journalism and exclusive events.