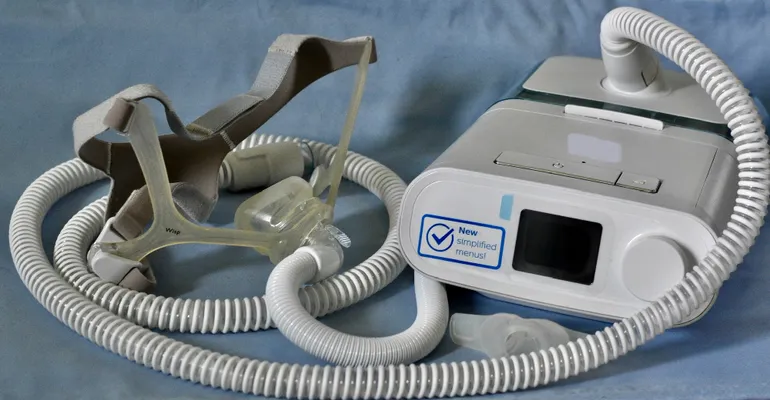
The Food and Drug Administration’s recall process was a key topic for the medical device industry in 2024.
At the start of the year, the U.S. Government Accountability Office said it would look at the agency’s recall process, following Philips’ recall of more than 15 million respiratory devices. The Philips recall, one of the largest in the industry’s history, has been ongoing since 2021. In April, Philips agreed to stop selling certain respiratory machines in the U.S. in a settlement with the FDA and the Department of Justice.
More recently, the FDA has taken actions to speed up public notices about recalls that it believes could be high risk. Under new Center for Devices and Radiological Health Director Michelle Tarver, the agency launched a pilot program in November and issued its first early alert on a Fresenius infusion pump recall this month.
Several heart devices have also come under scrutiny this year, including Abiomed’s Impella heart pumps and some of Abbott’s Heartmate devices. The Heartmate devices are the only left ventricular assist systems on the market after competitor Medtronic pulled its equivalent in 2021.
The FDA has also flagged quality issues with plastic syringes, stopping imports of the devices from certain manufacturers in China.
Here is MedTech Dive’s coverage of 2024’s most notable device recalls and product safety stories: