Dive Brief:
- Medtronic said Monday it received approval from the Food and Drug Administration for an extravascular defibrillator designed to treat abnormal heart rhythms and prevent sudden cardiac arrest, which can lead to death within minutes if not treated immediately.
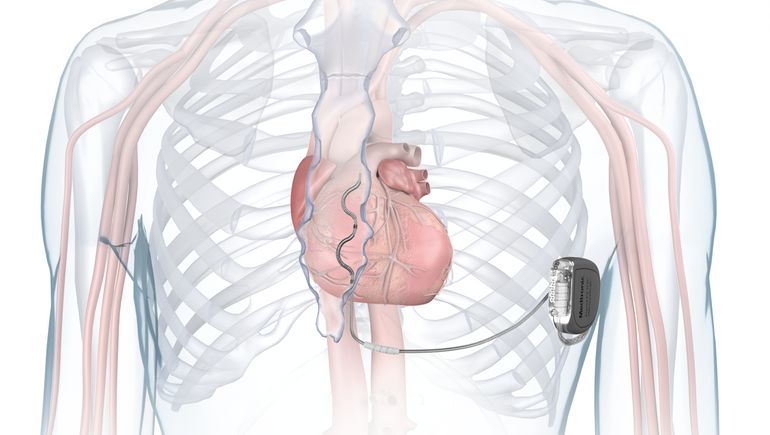
- Unlike traditional implantable cardioverter defibrillators, which have lead wires running between a pulse generator and the heart, Medtronic’s Aurora EV-ICD places a lead outside of the heart and veins.
- The Aurora EV-ICD was a PMA submission to the FDA, Medtronic spokesperson Tracy McNulty said in an email. “We estimate the current global EV-ICD market to be between $300-$350 million, and expect the EV-ICD market to reach $1 billion 10 years out from the Aurora launch,” McNulty said.
Dive Insight:
Although conventional ICDs have proved effective in preventing sudden cardiac death, complications with leads, including fractures and insulation failure, have prompted heart device makers to develop less-invasive approaches.
“Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology,” Bradley Knight, medical director of electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute, said in a statement provided by Medtronic.
Medtronic also makes a leadless pacemaker, called Micra, that is implanted directly into the heart to treat abnormally slow heart rhythms, while Abbott recently launched a leadless pacemaker called Aveir.
Analysts have said they expect Medtronic’s new extravascular ICD to compete with Boston Scientific’s subcutaneous implantable defibrillator, which gained FDA approval in 2012.
Medtronic said the Aurora EV-ICD is the first defibrillation system to treat dangerously fast heart rhythms with a lead placed under the breastbone, outside of the heart and veins. The system delivers anti-tachycardia pacing and back-up pause-prevention pacing via a device that is similar in size, shape and longevity to traditional transvenous ICDs.
FDA approval was supported by pivotal trial results, published in the New England Journal of Medicine, that demonstrated the system’s safety and effectiveness.
The device will be commercially available in the U.S. on a limited basis in the coming weeks, the company said.