Dive Brief:
- Medtronic said Monday it has received the first approval for a renal denervation system from China’s National Medical Products Administration (NMPA).
- The approval positions Medtronic to begin working through the provincial registration process and target some of the more than 245 million people in China with high blood pressure, according to the announcement.
- Medtronic expects sales in China of the Symplicity Spyral hypertension device to “be modest in the short-term.” Chinese companies are developing rival renal denervation systems.
Dive Insight:
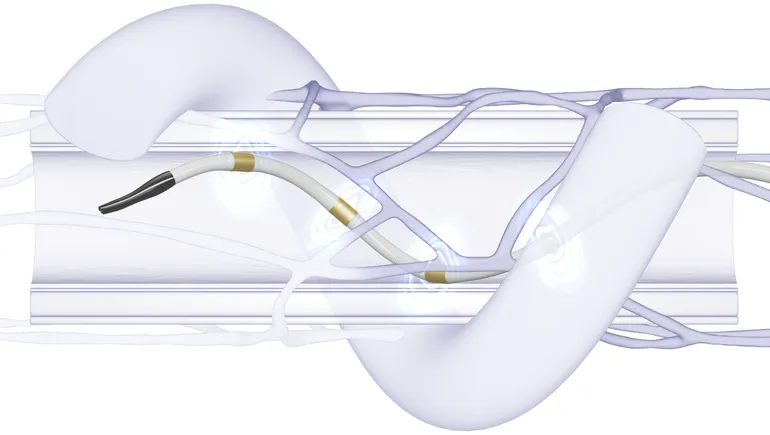
Medtronic’s renal denervation system is a catheter-based treatment to lower blood pressure by delivering radiofrequency energy to calm overactive nerves near the kidneys.
The company won FDA approval for Symplicity Spyral in November. The company received clearance to sell a renal denervation system in Europe more than 10 years ago but guidelines classed the approach as an investigational treatment until 2023. The European Society of Cardiology changed its stance in 2023 and recommended renal denervation in medication-resistant high blood pressure.
After unlocking the European and U.S. markets last year, Medtronic has now secured authorization in China. A recent study of hypertension in China, which Medtronic cited in the announcement, estimated that 245 million people in China have hypertension. Meanwhile, the World Health Organization said more than 270 million have hypertension, adding that only 14% have it under control and some people lack access to treatment.
Medtronic is expecting modest sales in the short term. NMPA approval is the first step in the process of getting a device to patients in China. Medtronic now plans to work through the provincial registration process.
Medtronic said it is the first company to take a renal denervation device through that process, but rivals have shown an interest in China. Recor Medical, which competes with Medtronic in the U.S., sold Otsuka Holdings the rights to commercialize its renal denervation technology in Asia in 2016. Otsuka acquired Recor two years later.
Chinese medtech companies are also developing renal denervation devices. In 2023, Symap Medical and Shanghai Golden Leaf Medtec published clinical trial data on their technologies. Investigators have also published data on Shanghai Wisegain Medical Devices’ system.