Dive Brief:
- Moon Surgical has obtained clearance from the Food and Drug Administration to sell the commercial version of its Maestro soft tissue surgical robot in the U.S., entering a market dominated by Intuitive Surgical’s da Vinci system.
- The company, with offices in Paris and San Francisco, is among a growing number of new competitors vying for a piece of a robotic surgery market that shows no signs of slowing.
- Moon plans a limited launch of the Maestro system in the U.S. and Europe before rolling out the robot more broadly in 2025, the company said on Wednesday.
Dive Insight:
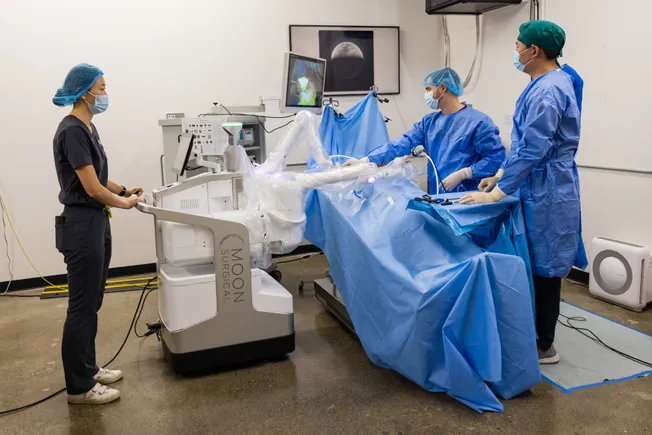
Moon sees a place in the market for its system to support laparoscopic surgeries that are not currently compatible with traditional robot-assisted devices. The company estimates that nearly 19 million additional soft tissue surgical procedures a year could be aided by the use of robotic systems.
In a laparoscopic procedure, surgical instruments are inserted into the body through small incisions. The minimally invasive approach can shorten patient recovery times.
Moon said its Maestro robot can be integrated into existing clinical workflows for any laparoscopic indication and can help improve operating room efficiency.
CEO Anne Osdoit said U.S. sites are “lining up” to participate in Maestro’s limited market release.
“Our Maestro System introduces a new category of robotic surgery tailored for the broad laparoscopy market, enabling robotics utilization on an unprecedented scale,” Osdoit said in a statement.
The system received a CE mark in September 2023 and has been used to treat more than 200 patients in general, bariatric and gynecologic surgery at two European pilot sites, the company said. In December 2022, Moon announced its first FDA clearance for a noncommercial version of Maestro.
Moon joins an expanding list of robot makers preparing to compete in the soft tissue surgery market. Among the latest entrants, Medical Microinstruments in April received the FDA’s de novo classification for its Symani system used in reconstructive microsurgery, and Virtual Incision gained a de novo authorization in February for its Mira robot in colectomy procedures.
As smaller companies try to catch up to Intuitive, the market leader is rolling out the latest version of its robot, da Vinci 5. The new system is Intuitive’s first in 10 years.