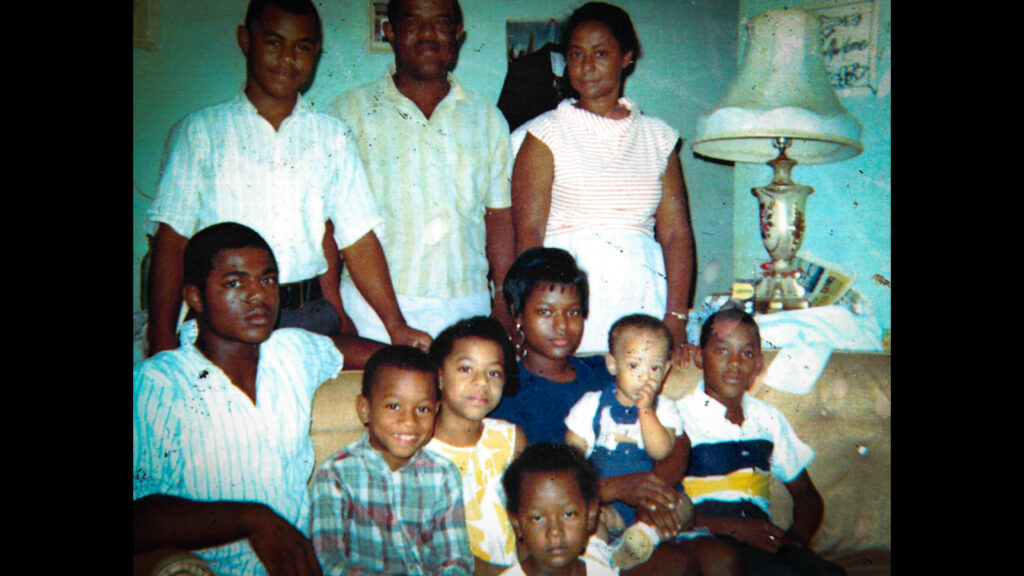I was born in Alabama in the Jim Crow South, one of eight siblings in a hard-working Black farming community. Our family doctor, a leading figure in the community, inspired me to follow in his footsteps and help people in need.
Years later, while working as a physician in Massachusetts, I was appalled to observe how many medicines failed to benefit society’s most vulnerable, in particular people of color. I soon shifted my career into drug development, because I felt I could improve health care on a bigger scale through innovation.
advertisement
In particular, I wanted to help patients with sickle cell disease, whom I’d seen endure terrible discrimination. Sickle cell is an inherited blood condition that leads to pain, organ failure, and early death. It affects some 100,000 people in the United States, 90% of whom are of African descent, and millions more around the world. Small children with the disease face high death rates. And yet the U.S. health care system failed to properly address it.
I helped build a company, Global Blood Therapeutics, in order to support the patients hardest hit by this devastating disease. In 2019, the Food and Drug Administration approved our once-a-day tablet, Oxbryta, the first sickle cell drug that treats the condition’s root cause. In 2022, we negotiated a profitable deal to sell our company to Pfizer, aiming to take advantage of the global company’s reach so that Oxbryta could benefit more patients — particularly in Africa, India, and South America.
Unfortunately, I fear that as a direct result of two recent policy shifts, we’ll soon hear fewer success stories of this kind.
advertisement
In May, the Federal Trade Commission sued to block Amgen from acquiring Horizon Therapeutics, making good on its earlier promise to “take an aggressive approach” with biopharma mergers. The deal may yet go through, but the lawsuit, combined with the agency’s new, more zealous way of doing things, will surely chill merger-and-acquisition activity.
That’s deeply unfortunate, because these arrangements are often crucial to expanding production and distribution capacity. Mergers and partnerships are critical to ensure resources to conduct expensive clinical trials, secure regulatory approval, and then manufacture and distribute medicine at a national or global scale. This type of deal-making is particularly important for small companies.
That was certainly the case at Global Blood Therapeutics. We could not have reached patients in lower-income countries, where child mortality from sickle cell disease is much greater, without being acquired by Pfizer.
Yet FTC officials have recently argued for greater oversight of pharma mergers and even suggested broadening the definition of anticompetitive behavior. If the FTC clamps down on mergers, it will push companies to build duplicate global distribution systems, which is inefficient at best and simply unaffordable for smaller businesses. Mergers like the Amgen-Horizon deal expand global access to medicines, plain and simple.
The other worrisome policy change comes from implementation of last year’s Inflation Reduction Act, which imposes government price setting on a range of popular drugs purchased by Medicare.
Biopharmaceutical companies already take enormous financial risks, in the hope that the few drugs that make it all the way to FDA approval — about 12% of those that enter clinical trials — will allow them to recoup expenses and fund future research. Now, the new provisions in the IRA put drug developers in a more financially precarious position, pressuring companies to reduce investment in research and development.
As if that weren’t bad enough, the IRA singles out small-molecule drugs — the pills that make up the majority of medicines and are often easier to administer than large-molecule biologic drugs, which are grown from living cells. Under the IRA, biologics are exempt from price setting for 13 years after FDA approval, but small-molecule drugs are exempt only for nine years.
This provision will encourage investors and drug companies to redeploy resources away from important small-molecule drugs, many of which have been crucial to advances in neurology and oncology. Biologics are vital, innovative medicines, but Congress shouldn’t be in the business of picking scientific winners and losers.
My company’s Oxbryta is a small-molecule drug. During the decade before we were acquired, Global Blood Therapeutics raised more than $1.5 billion and never turned a profit. That capital, in turn, allowed us to help a population long mistreated and ignored by the medical establishment. But I doubt this would have been possible if price controls had been looming — and it would have been even less likely with government policies that penalized the type of drugs that we developed.
The regulators and lawmakers behind these two changes say they want to bring down the cost of medicine, which is a noble intention. But the fact is, their actions will do more harm than good. Together, the new rules under the IRA and actions by the FTC are creating an atmosphere that is increasingly hostile to biotech entrepreneurship. That will quash research and investment into groundbreaking drugs. And it will ensure that more patients with overlooked or hard-to-treat conditions unnecessarily suffer for a longer time.
Dr. Ted W. Love is chair of the Biotechnology Innovation Organization’s Board of Directors and former president and CEO of Global Blood Therapeutics.