Kaplan is a researcher and an expert in clinical research standards. Weisman is a rheumatologist and a professor of medicine.
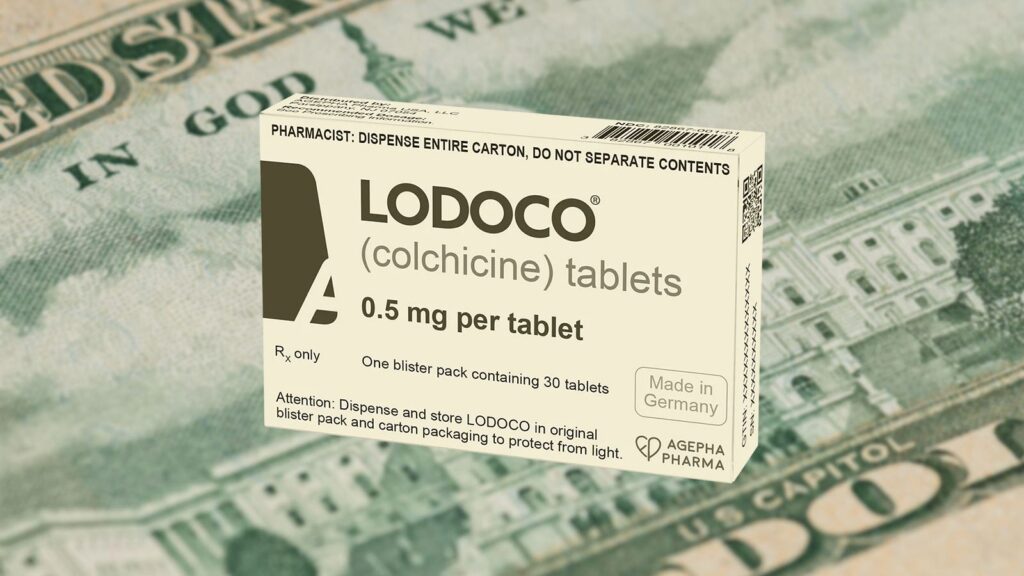
In June the U.S. FDA approved colchicine (Lodoco) as the first anti-inflammatory drug for the prevention of heart attacks and strokes among people with established heart disease or multiple risk factors. One recent review reported that inflammation is a better predictor of future cardiovascular events and death than elevated cholesterol. So, the availability of a medicine that addresses inflammation is good news. But the dark side to this approval is about money.
Colchicine is not a new drug. It has been used for centuries. In fact, there are reports it was used to treat gout in ancient Greece over 2,000 years ago and it has remained a mainstay in gout treatment. For decades, it has also been used to treat familial Mediterranean fever and pericarditis. Over the last few years, a significant number of studies have documented that colchicine reduces the risk of heart attacks and strokes among people with established heart disease. One large study published in 2019 followed heart disease patients for nearly 2 years and tabulated how many people died from cardiovascular causes, were resuscitated, experienced cardiac arrest, had a heart attack or stroke, or needed urgent hospitalization. In comparison to those randomly assigned to take a placebo, those taking colchicine were 23% less likely to experience one of these outcomes.
Overall, the benefits of colchicine look impressive. But here is the rub. Although colchicine has been used successfully for centuries, it had not been patented until relatively recently. Back in 2006, the FDA created the “unapproved drugs initiative” for older generic drugs, like colchicine, that had never been carefully evaluated by the FDA for safety and efficacy. Companies that invested in these evaluations were rewarded with the opportunity to patent the molecules. Immediately the pharmaceutical companies began doing pharmacokinetic and clinical studies on these older medications. Given the timing, we believe the primary goal was to bring them to market as high-cost patented brand-named products. In 2009, the FDA allowed URL Pharma to bring colchicine to market under the brand name Colcrys at a new dose of 0.6 mg per pill. Under the Orphan Drugs Act, they were given 3 years of market exclusivity at this dose for the treatment of gout. URL Pharma also got an additional 7 years to exclusively market Colcrys for the treatment of familial Mediterranean fever at the same dose of 0.6 mg per pill. As part of these agreements, unapproved single-ingredient colchicine could not be sold in the U.S. and disappeared from the market.
FDA and the public soon recognized that the unintended consequences of the initiative also included drug shortages and higher prices to treat gout. With these new approvals, the cost of colchicine accelerated from pennies to about $5 per pill. The rheumatology community and other groups were extremely upset when their patients could no longer obtain generic colchicine at what many prescribers viewed as a fair price. During the decade following the price increase, there was a 27% reduction in the use of colchicine among gout patients who needed it. In 2013, applications were filed (and later granted in several countries abroad) for new patents allowing colchicine to be marketed as a novel agent for the prevention of cardiovascular events. While there are now 17 patents for Colcrys, until last June there was only one FDA approved form of colchicine.
Agepha Pharma now holds eight patents on Lodoco (simply 0.5 mg of colchicine) for preventing heart disease or stroke. Now that the periods for exclusive marketing of Colcrys have expired, the price has come back down. Although the retail price remains around $5 per pill, large chain pharmacies sell it for less than $1/pill. Colcrys still remains FDA-approved for the treatment of gout at a dose of 0.6 mg per pill and generics remain unavailable.
Meanwhile, Agepha just released a retail price for Lodoco of $621 for a 30-day supply — nearly $21/pill. It will be available with a coupon at Walgreens for $170/month or about $5.66/pill. Using either the retail or coupon price, Lodoco can cost as much as four to six times more than Colcrys. Yet, Colcrys and Lodoco are essentially the same drug. Both have only one active ingredient — colchicine. The only difference is a tiny variation in the dose. Colcrys has 0.6 mg of colchicine, while Lodoco has 0.5 mg — a difference that could be considered of no clinical consequence. Doctors who want to use colchicine to prevent heart disease will be able to legally write off label prescriptions for the lower cost Colcrys. However, modern corporate medicine may deny payment because the dose of 0.6 mg was not approved for this indication (heart disease prevention). Insurers, regulators, or prescribers could theoretically argue that the higher dose was not tested for heart disease prevention and that 0.6 mg could be more toxic than 0.5 mg. Further, many physicians and healthcare provider groups avoid off label prescriptions because it may affect their quality ratings.
To be clear, we are strong supporters of the search for safe and effective medications. We recognize that the process requires significant investment. But colchicine has a long history of use and has established its value in several areas of medicine. The unaffordability of prescription drugs remains an important driver of uncontrollable healthcare costs. For those with inadequate resources or lack of health insurance, allowing this well-established generic medication to become patented and hence unaffordable to some has the potential to produce significant harm. We hope Congress and the FDA will take this issue seriously.
Robert M Kaplan, PhD, is a faculty member at Stanford University’s Clinical Excellence Research Center, a former associate director of the National Institutes of Health, and a former chief science officer for the U.S. Agency for Health Care Research and Quality. Michael H Weisman, MD, is a rheumatologist, a distinguished professor of medicine emeritus at David Geffen School of Medicine at UCLA, and an adjunct professor of medicine at Stanford University. He has served in advisory roles for the FDA and for the pharmaceutical industry. He has not been associated with companies making colchicine, Lodoco, Colcrys, or their competitors.
Please enable JavaScript to view the